当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High-Concentration Additive and Triiodide/Iodide Redox Couple Stabilize Lithium Metal Anode and Rejuvenate the Inactive Lithium in Carbonate-Based Electrolyte
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2022-06-22 , DOI: 10.1002/adfm.202204768 Zuxin Wen 1 , Wenqiang Fang 1 , Xiaoyu Wu 2 , Zuoyu Qin 1 , Hong Kang 1 , Long Chen 1 , Ning Zhang 1 , Xiaohe Liu 3 , Gen Chen 1
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2022-06-22 , DOI: 10.1002/adfm.202204768 Zuxin Wen 1 , Wenqiang Fang 1 , Xiaoyu Wu 2 , Zuoyu Qin 1 , Hong Kang 1 , Long Chen 1 , Ning Zhang 1 , Xiaohe Liu 3 , Gen Chen 1
Affiliation
|
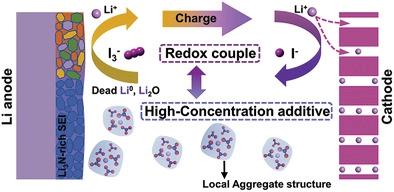
Carbonate-based electrolytes are incompatible with lithium (Li) metal anode because the generated solid electrolyte interphase (SEI) undergoes repeated breakage-repair, resulting in the accumulation of inactive Li including Li+ compounds and electrically isolated dead Li0 in the SEI. Therefore, exploiting a suitable strategy to construct a stable SEI while efficiently rejuvenating the inactive Li capacity is urgent and more thoughtful than just building a stereotyped SEI layer. Herein, an innovative strategy is proposed of high-concentration additive (HCA) of LiNO3 inspired by (localized) high-concentration electrolyte and inactive Li restoration methodology via triiodide/iodide (I3−/I−) redox couple to improve the compatibility of carbonate-based electrolytes. The HCA of LiNO3 can maintain the cation–anion aggregates solvation structures in the carbonate-based bulk electrolyte and induce the in situ formation of superior-ionic-conductivity NO3−-derived SEI. Moreover, the reversible I3−/I− redox couple can further optimize the SEI and constantly rejuvenate the inactive Li including solvent/LiNO3-derived Li2O, a derivative has almost been acquiescent in LiNO3-additive electrolytes, and dead Li0 into delithiated cathode. Consequently, epitaxy-like planar Li deposition, better reversibility, and higher capacity retention can be realized and are systematically verified by Li||Cu half cells, full cells with excess/limited Li (N/P ratio = 1.5) and anode-free lithium metal batteries.
中文翻译:

高浓度添加剂和三碘化物/碘化物氧化还原对稳定锂金属负极,使碳酸盐基电解液中的惰性锂恢复活力
碳酸盐基电解质与锂 (Li) 金属负极不相容,因为生成的固体电解质中间相 (SEI) 会经历反复的断裂修复,导致在 SEI 中积累包括 Li +化合物在内的惰性 Li 和电隔离的死 Li 0。因此,开发一种合适的策略来构建稳定的 SEI,同时有效地恢复非活性锂容量比仅仅构建一个刻板的 SEI 层更为紧迫和周到。在此,受(局部)高浓度电解质和通过三碘化物/碘化物(I 3 - /I -) 氧化还原对提高碳酸盐基电解质的相容性。LiNO 3的 HCA可以保持碳酸盐基体电解质中的阳离子-阴离子聚集体溶剂化结构,并诱导原位形成具有优异离子电导率的 NO 3 -衍生 SEI。此外,可逆的 I 3 - /I -氧化还原电对可以进一步优化 SEI 并不断恢复非活性锂,包括溶剂/LiNO 3衍生的 Li 2 O,该衍生物几乎默认用于添加 LiNO 3的电解质,而死锂0进入脱锂阴极。因此,可以实现类似外延的平面锂沉积、更好的可逆性和更高的容量保持率,并通过 Li||Cu 半电池、具有过量/有限 Li(N/P 比 = 1.5)和无阳极的全电池进行系统验证锂金属电池。
更新日期:2022-06-22
中文翻译:

高浓度添加剂和三碘化物/碘化物氧化还原对稳定锂金属负极,使碳酸盐基电解液中的惰性锂恢复活力
碳酸盐基电解质与锂 (Li) 金属负极不相容,因为生成的固体电解质中间相 (SEI) 会经历反复的断裂修复,导致在 SEI 中积累包括 Li +化合物在内的惰性 Li 和电隔离的死 Li 0。因此,开发一种合适的策略来构建稳定的 SEI,同时有效地恢复非活性锂容量比仅仅构建一个刻板的 SEI 层更为紧迫和周到。在此,受(局部)高浓度电解质和通过三碘化物/碘化物(I 3 - /I -) 氧化还原对提高碳酸盐基电解质的相容性。LiNO 3的 HCA可以保持碳酸盐基体电解质中的阳离子-阴离子聚集体溶剂化结构,并诱导原位形成具有优异离子电导率的 NO 3 -衍生 SEI。此外,可逆的 I 3 - /I -氧化还原电对可以进一步优化 SEI 并不断恢复非活性锂,包括溶剂/LiNO 3衍生的 Li 2 O,该衍生物几乎默认用于添加 LiNO 3的电解质,而死锂0进入脱锂阴极。因此,可以实现类似外延的平面锂沉积、更好的可逆性和更高的容量保持率,并通过 Li||Cu 半电池、具有过量/有限 Li(N/P 比 = 1.5)和无阳极的全电池进行系统验证锂金属电池。











































 京公网安备 11010802027423号
京公网安备 11010802027423号