当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Trapping an Oxidized and Protonated Intermediate of the [FeFe]-Hydrogenase Cofactor under Mildly Reducing Conditions
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2022-06-21 , DOI: 10.1021/acs.inorgchem.2c00954 Moritz Senger 1 , Jifu Duan 2 , Mariia V Pavliuk 1 , Ulf-Peter Apfel 3, 4 , Michael Haumann 5 , Sven T Stripp 6
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2022-06-21 , DOI: 10.1021/acs.inorgchem.2c00954 Moritz Senger 1 , Jifu Duan 2 , Mariia V Pavliuk 1 , Ulf-Peter Apfel 3, 4 , Michael Haumann 5 , Sven T Stripp 6
Affiliation
|
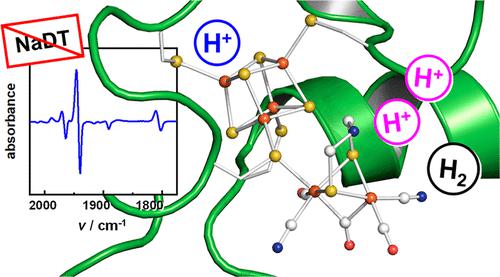
The H-cluster is the catalytic cofactor of [FeFe]-hydrogenase, a metalloenzyme that catalyzes the formation of dihydrogen (H2). The catalytic diiron site of the H-cluster carries two cyanide and three carbon monoxide ligands, making it an excellent target for IR spectroscopy. In previous work, we identified an oxidized and protonated H-cluster species, whose IR signature differs from that of the oxidized resting state (Hox) by a small but distinct shift to higher frequencies. This “blue shift” was explained by a protonation at the [4Fe–4S] subcomplex of the H-cluster. The novel species, denoted HoxH, was preferentially accumulated at low pH and in the presence of the exogenous reductant sodium dithionite (NaDT). When HoxH was reacted with H2, the hydride state (Hhyd) was formed, a key intermediate of [FeFe]-hydrogenase turnover. A recent publication revisited our protocol for the accumulation of HoxH in wild-type [FeFe]-hydrogenase, concluding that inhibition by NaDT decay products rather than cofactor protonation causes the spectroscopic “blue shift”. Here, we demonstrate that HoxH formation does not require the presence of NaDT (or its decay products), but accumulates also with the milder reductants tris(2-carboxyethyl)phosphine, dithiothreitol, or ascorbic acid, in particular at low pH. Our data consistently suggest that HoxH is accumulated when deprotonation of the H-cluster is impaired, thereby preventing the regain of the oxidized resting state Hox in the catalytic cycle.
中文翻译:

在轻度还原条件下捕获 [FeFe]-氢化酶辅因子的氧化和质子化中间体
H-簇是[FeFe]-氢化酶的催化辅助因子,氢化酶是一种催化二氢(H 2)形成的金属酶。H-簇的催化二铁位点带有两个氰化物和三个一氧化碳配体,使其成为红外光谱的极好目标。在以前的工作中,我们确定了一种氧化和质子化的 H 簇物种,其 IR 特征与氧化静息态 (Hox) 的特征不同,其向更高频率的转变很小但明显。这种“蓝移”可以通过 H 簇的 [4Fe-4S] 亚配合物的质子化来解释。新物种,表示为 HoxH,优先在低 pH 值和外源还原剂连二亚硫酸钠 (NaDT) 存在下积累。当 HoxH 与 H 2反应时,形成氢化物态(Hhyd),这是[FeFe]-氢化酶转换的关键中间体。最近的一份出版物重新审视了我们在野生型 [FeFe]-氢化酶中积累 HoxH 的方案,得出的结论是 NaDT 衰变产物而不是辅因子质子化的抑制会导致光谱“蓝移”。在这里,我们证明了 HoxH 的形成不需要 NaDT(或其衰变产物)的存在,但也与较温和的还原剂三(2-羧乙基)膦、二硫苏糖醇或抗坏血酸一起积累,特别是在低 pH 值下。我们的数据一致表明,当 H 簇的去质子化受损时,HoxH 会积累,从而防止在催化循环中恢复氧化的静息状态 Hox。
更新日期:2022-06-21
中文翻译:

在轻度还原条件下捕获 [FeFe]-氢化酶辅因子的氧化和质子化中间体
H-簇是[FeFe]-氢化酶的催化辅助因子,氢化酶是一种催化二氢(H 2)形成的金属酶。H-簇的催化二铁位点带有两个氰化物和三个一氧化碳配体,使其成为红外光谱的极好目标。在以前的工作中,我们确定了一种氧化和质子化的 H 簇物种,其 IR 特征与氧化静息态 (Hox) 的特征不同,其向更高频率的转变很小但明显。这种“蓝移”可以通过 H 簇的 [4Fe-4S] 亚配合物的质子化来解释。新物种,表示为 HoxH,优先在低 pH 值和外源还原剂连二亚硫酸钠 (NaDT) 存在下积累。当 HoxH 与 H 2反应时,形成氢化物态(Hhyd),这是[FeFe]-氢化酶转换的关键中间体。最近的一份出版物重新审视了我们在野生型 [FeFe]-氢化酶中积累 HoxH 的方案,得出的结论是 NaDT 衰变产物而不是辅因子质子化的抑制会导致光谱“蓝移”。在这里,我们证明了 HoxH 的形成不需要 NaDT(或其衰变产物)的存在,但也与较温和的还原剂三(2-羧乙基)膦、二硫苏糖醇或抗坏血酸一起积累,特别是在低 pH 值下。我们的数据一致表明,当 H 簇的去质子化受损时,HoxH 会积累,从而防止在催化循环中恢复氧化的静息状态 Hox。











































 京公网安备 11010802027423号
京公网安备 11010802027423号