Ultrasonics Sonochemistry ( IF 8.7 ) Pub Date : 2022-06-21 , DOI: 10.1016/j.ultsonch.2022.106074 Wen Li 1 , Wanchao Chen 2 , Haile Ma 3 , Di Wu 2 , Zhong Zhang 2 , Yan Yang 2
|
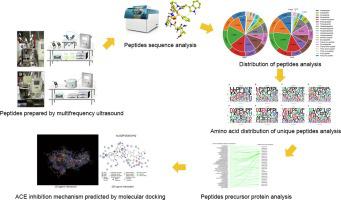
To reveal the structural characteristics and angiotensin-converting enzyme (ACE) inhibition mechanism of Stropharia rugosoannulata mushroom peptides prepared by multifrequency ultrasound, the peptide distribution, amino acid sequence composition characteristics, formation pathway, and ACE inhibition mechanism of S. rugosoannulata mushroom peptides were studied. It was found that the peptides in S. rugosoannulata mushroom samples treated by multifrequency ultrasound (probe ultrasound and bath ultrasound mode) were mainly octapeptides, nonapeptides, and decapeptides. Hydrophobic amino acids were the primary amino acids in the peptides prepared by ultrasound, and the amino acid dissociation of the peptide bonds at the C-terminal under the action of ultrasound was performed mainly to produce hydrophobic amino acids. Pro and Val (PV), Arg and Pro (RP), Pro and Leu (PL), and Asp (D) combined with hydrophobic amino acids were the characteristic amino acid sequence basis of the active peptides of the S. rugosoannulata mushroom. The docking results of active peptides and ACE showed that hydrogen bond interaction remained the primary mode of interaction between ACE and peptides prepared by ultrasound. The peptides can bind to the amino acid residues in the ACE active pocket, zinc ions, or key amino acids in the domain, and this results in inhibition of ACE activity. Cation–pi interactions also played an important role in the binding of mushroom peptides to ACE. This study explains the structural characteristics and ACE inhibition mechanism used by S. rugosoannulata mushroom peptides prepared by ultrasound, and it will provide a reference for the development and application of S. rugosoannulata mushroom peptides.
中文翻译:

超声波法制备毛茛蕈肽的结构表征及血管紧张素转化酶(ACE)抑制机制
为揭示多频超声法制备的大红菇肽的结构特征和血管紧张素转化酶(ACE)抑制机制,研究了大红菇肽的肽分布、氨基酸序列组成特征、形成途径和ACE抑制机制。 . 发现S. rugosoannulata中的肽经多频超声(探针超声和浴超声模式)处理的蘑菇样品主要为八肽、九肽和十肽。疏水性氨基酸是超声制备的多肽中的主要氨基酸,在超声作用下C端肽键的氨基酸解离主要产生疏水性氨基酸。Pro and Val (PV), Arg and Pro (RP), Pro and Leu (PL), Asp (D) 与疏水性氨基酸结合是茯苓活性肽的特征性氨基酸序列基础蘑菇。活性肽与ACE的对接结果表明,氢键相互作用仍然是ACE与超声制备肽相互作用的主要方式。这些肽可以与ACE活性口袋中的氨基酸残基、锌离子或结构域中的关键氨基酸结合,从而抑制ACE活性。阳离子-π 相互作用在蘑菇肽与 ACE 的结合中也发挥了重要作用。本研究解释了超声制备的茯苓多肽的结构特点和ACE抑制机制,为茯苓多肽的开发和应用提供参考。











































 京公网安备 11010802027423号
京公网安备 11010802027423号