当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Azobenzene Isomerization Investigation of Photoswitchable Glycomacrocycles
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-21 , DOI: 10.1021/acs.joc.2c00652 Jinbiao Jiao 1 , Stéphane Maisonneuve 1 , Juan Xie 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-21 , DOI: 10.1021/acs.joc.2c00652 Jinbiao Jiao 1 , Stéphane Maisonneuve 1 , Juan Xie 1
Affiliation
|
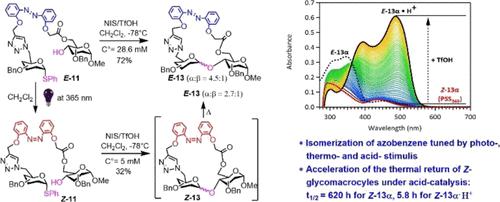
Macrocyclic glycoazobenzenes, as an emerging class of photoswitchable chiral macrocyclic compounds, have shown interesting properties since their discovery in 2017. We have recently employed the azobenzene-ester-linked glycosyl donor–acceptor pairs to study the influence of photoisomerization on intramolecular glycosylation. To continue the investigation on the stereoselectivity aspect of glycosylation and also to enlarge the diversity of photoswitchable glycomacrocycles, we have chosen azobenzene-triazole linkers in the present study and shown that the stereoselectivity of the glycosylation is dependent on the linker length, the configuration of the azobenzene template, as well as the reaction concentration. We have optimized the reaction conditions to prepare in good yields new glycomacrocycles, which displayed excellent photochromic properties. The influence of glycosylation reagents and acidity on the stability of the Z-azobenzene substrates and cyclic glycoazobenzenes has also been investigated, demonstrating that isomerization of macrocyclic azobenzene can be tuned by photo-, thermo-, and acid stimulus.
中文翻译:

光开关糖大环化合物的合成及偶氮苯异构化研究
大环氨基偶氮苯作为一类新兴的光可切换手性大环化合物,自 2017 年发现以来已显示出有趣的特性。我们最近采用偶氮苯-酯连接的糖基供体-受体对来研究光异构化对分子内糖基化的影响。为了继续研究糖基化的立体选择性方面并扩大光可切换糖大环的多样性,我们在本研究中选择了偶氮苯-三唑接头,并表明糖基化的立体选择性取决于接头长度、结构偶氮苯模板,以及反应浓度。我们优化了反应条件,以高产率制备了具有优异光致变色性能的新型糖大环化合物。还研究了Z-偶氮苯底物和环状氨基偶氮苯,证明大环偶氮苯的异构化可以通过光、热和酸刺激来调节。
更新日期:2022-06-21
中文翻译:

光开关糖大环化合物的合成及偶氮苯异构化研究
大环氨基偶氮苯作为一类新兴的光可切换手性大环化合物,自 2017 年发现以来已显示出有趣的特性。我们最近采用偶氮苯-酯连接的糖基供体-受体对来研究光异构化对分子内糖基化的影响。为了继续研究糖基化的立体选择性方面并扩大光可切换糖大环的多样性,我们在本研究中选择了偶氮苯-三唑接头,并表明糖基化的立体选择性取决于接头长度、结构偶氮苯模板,以及反应浓度。我们优化了反应条件,以高产率制备了具有优异光致变色性能的新型糖大环化合物。还研究了Z-偶氮苯底物和环状氨基偶氮苯,证明大环偶氮苯的异构化可以通过光、热和酸刺激来调节。











































 京公网安备 11010802027423号
京公网安备 11010802027423号