当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible-Light-Driven Organophotocatalyzed Multicomponent Approach for Tandem C(sp3)–H Activation and Alkylation Followed by Trifluoromethylthiolation
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-21 , DOI: 10.1021/acs.joc.2c00783 Krishna Gopal Ghosh 1 , Debabrata Das 1 , Sumit Garai 1 , Palasetty Chandu 1 , Devarajulu Sureshkumar 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-21 , DOI: 10.1021/acs.joc.2c00783 Krishna Gopal Ghosh 1 , Debabrata Das 1 , Sumit Garai 1 , Palasetty Chandu 1 , Devarajulu Sureshkumar 1
Affiliation
|
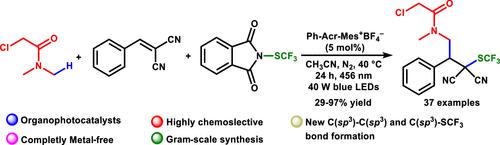
A visible-light-driven organophotocatalyzed multicomponent approach has been developed for tandem direct C(sp3)–H activation and alkylation followed by trifluoromethylthiolation in a one-pot operation. We report a completely metal-free, tandem, three-component approach for the difunctionalization of activated alkenes via the photoinduced radical pathway. This protocol allows the formation of two new C(sp3)–C(sp3) and C(sp3)–SCF3 bonds using a bench-stable, easy-to-handle trifluoromethylthiolating reagent under mild reaction conditions. The generosity of this reaction is shown with a library of C(sp3)–H donors and alkenes derivatives. The reaction conditions can tolerate a wide variety of functional groups. Gram-scale synthesis using environmentally benign and straightforward conditions highlights the synthetic advancement of the methodology. Further functionalization of the final product is also successfully demonstrated.
中文翻译:

可见光驱动的有机光催化多组分串联 C(sp3)-H 活化和烷基化后三氟甲基硫醇化方法
已经开发了一种可见光驱动的有机光催化多组分方法,用于串联直接 C(sp 3 )-H 活化和烷基化,然后在一锅操作中进行三氟甲基硫醇化。我们报告了一种完全无金属、串联、三组分的方法,用于通过光诱导自由基途径对活化烯烃进行双功能化。该协议允许在温和的反应条件下使用稳定、易于处理的三氟甲基硫醇化试剂形成两个新的 C(sp 3 )–C(sp 3 ) 和 C(sp 3 )–SCF 3键。该反应的慷慨性通过 C(sp 3)–H 供体和烯烃衍生物。反应条件可以容忍多种官能团。使用环境友好和直接条件的革兰氏规模合成突出了该方法的合成进步。最终产品的进一步功能化也得到了成功证明。
更新日期:2022-06-21
中文翻译:

可见光驱动的有机光催化多组分串联 C(sp3)-H 活化和烷基化后三氟甲基硫醇化方法
已经开发了一种可见光驱动的有机光催化多组分方法,用于串联直接 C(sp 3 )-H 活化和烷基化,然后在一锅操作中进行三氟甲基硫醇化。我们报告了一种完全无金属、串联、三组分的方法,用于通过光诱导自由基途径对活化烯烃进行双功能化。该协议允许在温和的反应条件下使用稳定、易于处理的三氟甲基硫醇化试剂形成两个新的 C(sp 3 )–C(sp 3 ) 和 C(sp 3 )–SCF 3键。该反应的慷慨性通过 C(sp 3)–H 供体和烯烃衍生物。反应条件可以容忍多种官能团。使用环境友好和直接条件的革兰氏规模合成突出了该方法的合成进步。最终产品的进一步功能化也得到了成功证明。










































 京公网安备 11010802027423号
京公网安备 11010802027423号