当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Friedel–Crafts Alkylation Reaction of Pyrroles with N-Unprotected Alkynyl Trifluoromethyl Ketimines
Organic Letters ( IF 4.9 ) Pub Date : 2022-06-21 , DOI: 10.1021/acs.orglett.2c01972 Tatsuhiro Uchikura 1 , Kureha Aruga 1 , Riku Suzuki 1 , Takahiko Akiyama 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-06-21 , DOI: 10.1021/acs.orglett.2c01972 Tatsuhiro Uchikura 1 , Kureha Aruga 1 , Riku Suzuki 1 , Takahiko Akiyama 1
Affiliation
|
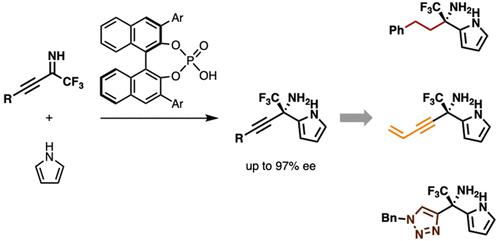
Developed herein is an enantioselective Friedel–Crafts alkylation reaction of N-unprotected alkynyl trifluoromethyl ketimines with pyrroles catalyzed by chiral phosphoric acid to furnish chiral primary α-trifluoromethyl-α-(2-pyrrolyl)propargylamines with high enantioselectivity. Transformation of the alkynyl group of the adducts afforded optically active α-trifluoromethylated amines bearing various substituents such as alkyl, alkenyl, enyne, and triazole without loss of optical purity.
中文翻译:

吡咯与 N-未保护的炔基三氟甲基酮亚胺的对映选择性 Friedel-Crafts 烷基化反应
本文开发了N-未保护的炔基三氟甲基酮亚胺与吡咯在手性磷酸催化下的对映选择性 Friedel-Crafts 烷基化反应,以提供具有高对映选择性的手性伯 α-三氟甲基-α-(2-吡咯基)炔丙基胺。加合物的炔基的转变提供了具有各种取代基(例如烷基、烯基、烯炔和三唑)的光学活性α-三氟甲基化胺,而不会损失光学纯度。
更新日期:2022-06-21
中文翻译:

吡咯与 N-未保护的炔基三氟甲基酮亚胺的对映选择性 Friedel-Crafts 烷基化反应
本文开发了N-未保护的炔基三氟甲基酮亚胺与吡咯在手性磷酸催化下的对映选择性 Friedel-Crafts 烷基化反应,以提供具有高对映选择性的手性伯 α-三氟甲基-α-(2-吡咯基)炔丙基胺。加合物的炔基的转变提供了具有各种取代基(例如烷基、烯基、烯炔和三唑)的光学活性α-三氟甲基化胺,而不会损失光学纯度。









































 京公网安备 11010802027423号
京公网安备 11010802027423号