Journal of Catalysis ( IF 6.5 ) Pub Date : 2022-06-20 , DOI: 10.1016/j.jcat.2022.06.023 Carrie A. Farberow , Evan C. Wegener , Anurag Kumar , Jacob H. Miller , Daniel P. Dupuis , Seonah Kim , Daniel A. Ruddy
|
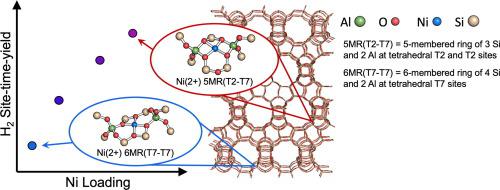
Ni-modified beta zeolite (Ni/BEA) catalysts activate carbon-hydrogen bonds in light alkanes, as demonstrated through isobutane reaction testing. Controlled synthesis of Ni/BEA allows for efficient introduction of ion-exchanged Ni sites at varying Ni loadings (0.43% − 1.8%). These catalysts exhibit site time yields (STY) for H2 production that increase with increasing Ni loading. A detailed analysis of secondary reactions and carbon deposition based on the relative molar flowrates of product C and H indicates that the observed increase in H2 STY with increasing Ni loading is likely attributed to both increasing alkane activation activity and increasing formation of hydrogen-deficient aromatic products retained within the catalyst pores. In situ diffuse-reflectance UV–visible-NIR absorbance and X-ray absorption spectroscopies indicate isolated, 4-coordinate Ni(2+) species for all loadings. Quantum mechanics/molecular mechanics modeling identifies two distinct Ni(2+) sites consistent with the structural characterization, but with differing relative stabilities due to their coordination environment. Computed reaction energetics for isobutane dehydrogenation demonstrate that the more stable Ni(2+) species at a six-membered 4Si-2Al ring, Ni-6MR, is less active for isobutane dehydrogenation than the less stable Ni(2+) at the five-membered 3Si-2Al ring, Ni-5MR. The differing local structure of the isolated cationic Ni sites in Ni/BEA offers a possible rationalization for the increased H2 STY observed at greater Ni loadings.
中文翻译:

将阳离子位点位置与 Ni/BEA 催化剂中的烷烃脱氢活性联系起来
正如异丁烷反应测试所证明的那样,镍改性的 β 沸石 (Ni/BEA) 催化剂可激活轻质烷烃中的碳氢键。Ni/BEA 的受控合成允许在不同的 Ni 负载 (0.43% - 1.8%) 下有效地引入离子交换的 Ni 位点。这些催化剂表现出随着Ni负载量增加而增加的H 2生产的位时产率(STY)。基于产物 C 和 H 的相对摩尔流量对二次反应和碳沉积的详细分析表明,观察到的 H 2增加增加 Ni 负载的 STY 可能归因于增加的烷烃活化活性和增加保留在催化剂孔内的缺氢芳烃产物的形成。原位漫反射 UV-可见-NIR 吸收和 X 射线吸收光谱表明所有负载的分离的 4 坐标 Ni(2+) 物种。量子力学/分子力学建模确定了两个与结构表征一致的不同 Ni(2+) 位点,但由于它们的配位环境而具有不同的相对稳定性。计算的异丁烷脱氢反应能量学表明,六元 4Si-2Al 环上更稳定的 Ni(2+) 物质 Ni-6MR 对异丁烷脱氢的活性低于五元环上不太稳定的 Ni(2+)成员 3Si-2Al 环,Ni-5MR。2 STY 在更大的镍负载下观察到。











































 京公网安备 11010802027423号
京公网安备 11010802027423号