Chem Catalysis ( IF 11.5 ) Pub Date : 2022-06-20 , DOI: 10.1016/j.checat.2022.05.023 Lei-Lei Qian , Yan-Cheng Hu , Xiang-Ting Min , Sa-Na Yang , Bing-Xue Shen , Boshun Wan , Qing-An Chen
|
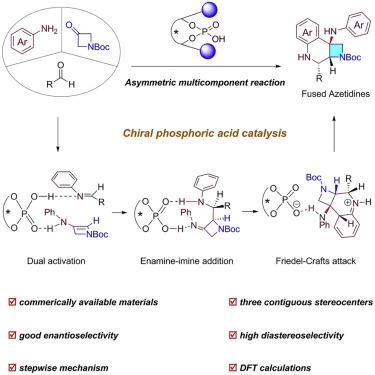
The wide occurrence of enantiopure-fused azetidines in various bioactive molecules leads to a great demand for their efficient synthetic methods. However, so far, organocatalytic protocols have been rather limited. Here we develop a chiral phosphoric acid (CPA)-catalyzed multicomponent reaction of anilines, aldehydes, and azetidinones to access tetrahydroquinoline-fused azetidines with three contiguous stereocenters. Noteworthy features include complete diastereocontrol, high enantioselectivity, good yields, and broad functional group tolerance. Successful implementation of this strategy relies on dual activation of imine and enamine intermediates with CPA. This work not only contributes an efficient organocatalytic assembly of chiral fused azetidines but also provides a paradigm for designing other asymmetric multicomponent reactions.
中文翻译:

CPA 催化的苯胺、醛和氮杂环丁酮的多组分反应:快速获得对映体融合的氮杂环丁烷
在各种生物活性分子中广泛存在的对映体融合氮杂环丁烷导致对其高效合成方法的巨大需求。然而,到目前为止,有机催化方案相当有限。在这里,我们开发了手性磷酸 (CPA) 催化的苯胺、醛和氮杂环丁酮的多组分反应,以获得具有三个连续立体中心的四氢喹啉稠合氮杂环丁烷。值得注意的特点包括完全的非对映控制、高对映选择性、良好的产率和广泛的官能团耐受性。该策略的成功实施依赖于亚胺和烯胺中间体与 CPA 的双重活化。这项工作不仅有助于手性稠合氮杂环丁烷的有效有机催化组装,而且还为设计其他不对称多组分反应提供了范例。










































 京公网安备 11010802027423号
京公网安备 11010802027423号