当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Metal-Templated Route to Amino Acids with 3-Spiropyrrolidine Oxindole Core via a 1,3-Dipolar Addition of Azomethine Ylides to a Chiral Dehydroalanine Ni(II) Complex
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-06-20 , DOI: 10.1002/adsc.202200446 Zalina Gugkaeva 1 , Maria Panova 2 , Alexander Smolyakov 3 , Michael Medvedev 2 , Alan Tsaloev 4 , Ivan Godovikov 1 , Victor I. Maleev 3 , Vladimir Larionov 5
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-06-20 , DOI: 10.1002/adsc.202200446 Zalina Gugkaeva 1 , Maria Panova 2 , Alexander Smolyakov 3 , Michael Medvedev 2 , Alan Tsaloev 4 , Ivan Godovikov 1 , Victor I. Maleev 3 , Vladimir Larionov 5
Affiliation
|
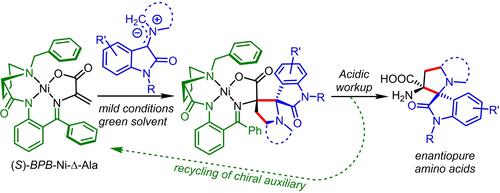
We herein developed a protocol for the asymmetric synthesis of artificial AAs featuring a 3-spiropyrrolidine oxindole skeletal with continuous tetrasubstituted carbon stereocenters by a 1,3-dipolar cycloaddition reaction of in situ generated azomethine ylides with a chiral dehydroalanine Ni(II) complex. A three-component reaction of the Ni(II) complex with various isatins and AAs in green solvent (ethanol) provided series of diastereomeric complexes with high dr (>20:1) in 40–86% yields. The formation of different regioisomers in the case of sarcosine and proline was explained using quantum chemical calculation. The acidic decomposition of the obtained Ni(II) complexes led to the target unnatural complex AAs with a 3-spiropyrrolidine oxindole core. The chiral auxiliary ligand was recovered after decomposition and reused for the synthesis of the starting dehydroalanine complex-substrate.
中文翻译:

通过 1,3-偶极加成甲亚胺叶立德到手性脱氢丙氨酸 Ni(II) 配合物,获得具有 3-螺吡咯烷氧吲哚核心的氨基酸的不对称金属模板途径
我们在此开发了一种不对称合成人工 AAs 的方案,该方案具有 3-螺吡咯烷氧吲哚骨架,通过 1,3-偶极环加成反应原位生成的偶氮甲碱叶立德与手性脱氢丙氨酸 Ni (II) 配合物进行 1,3-偶极环加成反应。Ni(II) 配合物与各种靛红和 AA 在绿色溶剂(乙醇)中的三组分反应提供了一系列具有高应力的非对映体配合物(>20:1),产率为 40–86%。使用量子化学计算解释了肌氨酸和脯氨酸不同区域异构体的形成。获得的 Ni(II) 配合物的酸性分解导致目标非天然配合物 AAs 具有 3-螺吡咯烷氧吲哚核心。分解后回收手性辅助配体并重新用于合成起始脱氢丙氨酸络合物-底物。
更新日期:2022-06-20
中文翻译:

通过 1,3-偶极加成甲亚胺叶立德到手性脱氢丙氨酸 Ni(II) 配合物,获得具有 3-螺吡咯烷氧吲哚核心的氨基酸的不对称金属模板途径
我们在此开发了一种不对称合成人工 AAs 的方案,该方案具有 3-螺吡咯烷氧吲哚骨架,通过 1,3-偶极环加成反应原位生成的偶氮甲碱叶立德与手性脱氢丙氨酸 Ni (II) 配合物进行 1,3-偶极环加成反应。Ni(II) 配合物与各种靛红和 AA 在绿色溶剂(乙醇)中的三组分反应提供了一系列具有高应力的非对映体配合物(>20:1),产率为 40–86%。使用量子化学计算解释了肌氨酸和脯氨酸不同区域异构体的形成。获得的 Ni(II) 配合物的酸性分解导致目标非天然配合物 AAs 具有 3-螺吡咯烷氧吲哚核心。分解后回收手性辅助配体并重新用于合成起始脱氢丙氨酸络合物-底物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号