当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tracer diffusion in proton-exchanged congruent LiNbO3 crystals as a function of hydrogen content
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2022-06-20 , DOI: 10.1039/d2cp01818g Lars Dörrer 1, 2 , René Heller 3 , Harald Schmidt 1, 2
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2022-06-20 , DOI: 10.1039/d2cp01818g Lars Dörrer 1, 2 , René Heller 3 , Harald Schmidt 1, 2
Affiliation
|
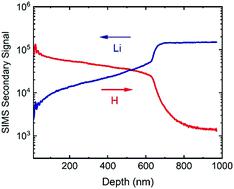
The proton-exchange process is an effective method of fabricating low-loss waveguides based on LiNbO3 crystals. During proton-exchange, lithium is replaced by hydrogen and Li1−xHxNbO3 is formed. Currently, mechanisms and kinetics of the proton-exchange process are unclear, primarily due to a lack in reliable tracer diffusion data. We studied lithium and hydrogen tracer diffusion in proton-exchanged congruent LiNbO3 single crystals in the temperature range between 130–230 °C. Proton-exchange was done in benzoic acid with 0, 1, 2, or 3.6 mol% lithium benzoate added, resulting in micrometre thick surface layers where Li is substituted by H with relative fractions between x = 0.45 and 0.85 as determined by Nuclear Reaction Analysis. For the diffusion experiments, ion-beam sputtered isotope enriched 6LiNbO3 was used as a Li tracer source and deuterated benzoic acid as a H tracer source. Isotope depth profile analysis was carried out by secondary ion mass spectrometry. From the experimental results, effective diffusivities governing the lithium/hydrogen exchange as well as individual hydrogen and lithium tracer diffusivities are extracted. All three types of diffusivities can be described by the Arrhenius law with an activation enthalpy of about 1.0–1.2 eV and increase as a function of hydrogen content nearly independent of temperature. The effective diffusivities and the lithium tracer diffusivities are identical within a factor of two to five, while the hydrogen diffusivities are higher by three orders of magnitude. The results show that the diffusion of Li is the rate determining step governing the proton-exchange process. Exponential dependencies between diffusivities and hydrogen concentrations are determined. The observed increase of Li tracer diffusivities and effective diffusivities as a function of hydrogen concentration is attributed to a continuous reduction of the migration enthalpy of diffusion by a maximum factor of about 0.2 eV. Simulations based on the determined diffusivities can reproduce the step-like profile of hydrogen penetration during proton-exchange.
中文翻译:

质子交换全等 LiNbO3 晶体中示踪剂扩散与氢含量的关系
质子交换工艺是制造基于LiNbO 3晶体的低损耗波导的有效方法。在质子交换过程中,锂被氢取代并形成Li 1- x H x NbO 3。目前,质子交换过程的机制和动力学尚不清楚,主要是由于缺乏可靠的示踪剂扩散数据。我们研究了在 130–230 °C 温度范围内质子交换全等 LiNbO 3单晶中的锂和氢示踪剂扩散。在添加 0、1、2 或 3.6 mol% 苯甲酸锂的苯甲酸中进行质子交换,产生微米厚的表面层,其中 Li 被 H 取代,相对分数在x之间= 0.45 和 0.85,由核反应分析确定。对于扩散实验,离子束溅射同位素富集6 LiNbO 3用作 Li 示踪源,氘代苯甲酸用作 H 示踪源。通过二次离子质谱法进行同位素深度剖面分析。从实验结果中,提取了控制锂/氢交换的有效扩散率以及单独的氢和锂示踪剂扩散率。所有三种类型的扩散率都可以用阿累尼乌斯定律来描述,活化焓约为 1.0-1.2 eV,并且随着氢含量的增加而增加,几乎与温度无关。有效扩散率和锂示踪剂扩散率在 2 到 5 倍范围内相同,而氢扩散率高出三个数量级。结果表明,Li的扩散是控制质子交换过程的速率决定步骤。确定了扩散率和氢浓度之间的指数相关性。观察到的锂示踪剂扩散率和有效扩散率随氢浓度的增加归因于扩散的迁移焓持续减少约 0.2 eV 的最大因子。基于确定的扩散率的模拟可以再现质子交换过程中氢渗透的阶梯状分布。
更新日期:2022-06-24
中文翻译:

质子交换全等 LiNbO3 晶体中示踪剂扩散与氢含量的关系
质子交换工艺是制造基于LiNbO 3晶体的低损耗波导的有效方法。在质子交换过程中,锂被氢取代并形成Li 1- x H x NbO 3。目前,质子交换过程的机制和动力学尚不清楚,主要是由于缺乏可靠的示踪剂扩散数据。我们研究了在 130–230 °C 温度范围内质子交换全等 LiNbO 3单晶中的锂和氢示踪剂扩散。在添加 0、1、2 或 3.6 mol% 苯甲酸锂的苯甲酸中进行质子交换,产生微米厚的表面层,其中 Li 被 H 取代,相对分数在x之间= 0.45 和 0.85,由核反应分析确定。对于扩散实验,离子束溅射同位素富集6 LiNbO 3用作 Li 示踪源,氘代苯甲酸用作 H 示踪源。通过二次离子质谱法进行同位素深度剖面分析。从实验结果中,提取了控制锂/氢交换的有效扩散率以及单独的氢和锂示踪剂扩散率。所有三种类型的扩散率都可以用阿累尼乌斯定律来描述,活化焓约为 1.0-1.2 eV,并且随着氢含量的增加而增加,几乎与温度无关。有效扩散率和锂示踪剂扩散率在 2 到 5 倍范围内相同,而氢扩散率高出三个数量级。结果表明,Li的扩散是控制质子交换过程的速率决定步骤。确定了扩散率和氢浓度之间的指数相关性。观察到的锂示踪剂扩散率和有效扩散率随氢浓度的增加归因于扩散的迁移焓持续减少约 0.2 eV 的最大因子。基于确定的扩散率的模拟可以再现质子交换过程中氢渗透的阶梯状分布。











































 京公网安备 11010802027423号
京公网安备 11010802027423号