当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The underappreciated influence of ancillary halide on metal–ligand proton tautomerism
Chemical Science ( IF 7.6 ) Pub Date : 2022-06-20 , DOI: 10.1039/d2sc00279e Anant Kumar Jain 1 , Michael R Gau 1 , Patrick J Carroll 1 , Karen I Goldberg 1
Chemical Science ( IF 7.6 ) Pub Date : 2022-06-20 , DOI: 10.1039/d2sc00279e Anant Kumar Jain 1 , Michael R Gau 1 , Patrick J Carroll 1 , Karen I Goldberg 1
Affiliation
|
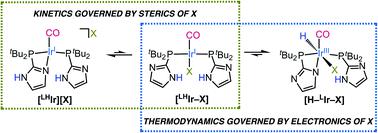
Syntheses of Vaska-type complexes [IrP2X(CO)] (P = phosphine, X = halide) with all four common halides (fluoride, chloride, bromide, and iodide) was attempted using a protic and hemilabile imidazolyl di-tert-butyl phosphine ligand. In the solid-state, all four complexes were found to be ionic with the halides in the outer-sphere, and the fourth coordination site of the square plane occupied by the imidazole arm of the ligand. In solution, however, the chloride complex was found to be in equilibrium with an octahedral IrIII–H species at room temperature. For the bromide and iodide analogs, the corresponding IrIII–H species were also observed but only after heating the solutions. The neutral IrI Vaska's analogs for X = Cl, Br, and I were obtained upon addition of excess halide salt, albeit heating was required for X = Br and I. The IrIII–H species are proposed to originate from tautomerization of minor amounts of the electron rich neutral Vaska analog (halide inner-sphere and phosphines monodentate) that are in equilibrium with the ionic species. Heating is required for the larger anions of bromide and iodide to overcome a kinetic barrier associated with their movement to an inner-sphere position prior to tautormerization. For the fluoride analog, the IrIII–H was not observed, attributable to strong hydrogen bonding interactions of the imidazolyl proton with the fluoride anion.
中文翻译:

辅助卤化物对金属-配体质子互变异构的未被充分认识的影响
Vaska 型配合物 [IrP 2 X(CO)] (P = 磷化氢,X = 卤化物) 与所有四种常见卤化物(氟化物、氯化物、溴化物和碘化物)的合成尝试使用质子和半可比咪唑基二叔-丁基膦配体。在固态下,发现所有四种配合物都与外层的卤化物呈离子状态,并且配体的咪唑臂占据了方形平面的第四个配位点。然而,在溶液中,发现氯化物络合物在室温下与八面体 Ir III -H 物质处于平衡状态。对于溴化物和碘化物类似物,也观察到相应的 Ir III -H 物质,但仅在加热溶液后。中性 Ir IVaska 的 X = Cl、Br 和 I 的类似物是在添加过量卤化物盐后获得的,尽管 X = Br 和 I 需要加热。Ir III -H 物种被认为源自少量富电子的互变异构化与离子物质平衡的中性 Vaska 类似物(卤化物内球和膦单齿)。较大的溴化物和碘化物阴离子需要加热,以克服与它们在互变异构化之前移动到内球位置相关的动力学障碍。对于氟化物类似物,没有观察到 Ir III -H,这归因于咪唑基质子与氟化物阴离子的强氢键相互作用。
更新日期:2022-06-20
中文翻译:

辅助卤化物对金属-配体质子互变异构的未被充分认识的影响
Vaska 型配合物 [IrP 2 X(CO)] (P = 磷化氢,X = 卤化物) 与所有四种常见卤化物(氟化物、氯化物、溴化物和碘化物)的合成尝试使用质子和半可比咪唑基二叔-丁基膦配体。在固态下,发现所有四种配合物都与外层的卤化物呈离子状态,并且配体的咪唑臂占据了方形平面的第四个配位点。然而,在溶液中,发现氯化物络合物在室温下与八面体 Ir III -H 物质处于平衡状态。对于溴化物和碘化物类似物,也观察到相应的 Ir III -H 物质,但仅在加热溶液后。中性 Ir IVaska 的 X = Cl、Br 和 I 的类似物是在添加过量卤化物盐后获得的,尽管 X = Br 和 I 需要加热。Ir III -H 物种被认为源自少量富电子的互变异构化与离子物质平衡的中性 Vaska 类似物(卤化物内球和膦单齿)。较大的溴化物和碘化物阴离子需要加热,以克服与它们在互变异构化之前移动到内球位置相关的动力学障碍。对于氟化物类似物,没有观察到 Ir III -H,这归因于咪唑基质子与氟化物阴离子的强氢键相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号