Water Research ( IF 11.4 ) Pub Date : 2022-06-18 , DOI: 10.1016/j.watres.2022.118778 Meng Li 1 , Yu-Ting Jin 2 , Dan-Yang Cao 3 , Ling-Ling Yang 3 , Jian-Fang Yan 3 , Zhao-Xin Zhang 4 , Zhang Liu 5 , Long-Wei Huang 2 , Shao-Qi Zhou 6 , Ji-Liang Cheng 3 , Qinglan Zhao 7 , Hai-Ming Zhao 3 , Nai-Xian Feng 3 , Ce-Hui Mo 3
|
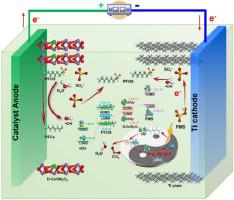
The electrochemical oxidation method is a promising technology for the degradation of perfluorooctane sulfonate (PFOS). However, the elimination processes of PFOS are still unknown, including the electron transfer pathway, key reactive sites, and degradation mechanism. Here, we fabricated diatomite and cerium (Ce) co-modified Sb2O3 (D-Ce/Sb2O3) anode to realize efficient degradation of PFOS via peroxymonosulfate (PMS) activation. The transferred electron and the generated hydroxyl radical (•OH) can high-effectively decompose PFOS. The electron can be rapidly transferred from the highest occupied molecular orbital of the PFOS to the lowest unoccupied molecular orbital of the PMS via the D-Ce/Sb2O3 driven by a potential energy difference under electrochemical process. The active site of Ce-O in the D-Ce/Sb2O3 can greatly reduce the migration distance of the electron and the •OH, and thus improving the catalytic activity for degrading various organic micropollutants with high stability. In addition, the electrochemical process shows strong resistance and tolerance to the changing pH, inorganic ions, and organic matter. This study offers insights into the electron transfer pathway and PMS activation mechanism in PFOS removal via electrochemical oxidation, paving the way for its potential application in water purification.
中文翻译:

水溶液中过氧单硫酸盐电化学活化高效分解全氟辛烷磺酸:功效、反应机理、活性位点和应用潜力
电化学氧化法是一种很有前景的降解全氟辛烷磺酸盐(PFOS)的技术。然而,PFOS的消除过程仍然未知,包括电子转移途径、关键反应位点和降解机制。在这里,我们制造了硅藻土和铈 (Ce) 共修饰的 Sb 2 O 3 (D-Ce/Sb 2 O 3 ) 阳极,以通过过一硫酸盐 (PMS) 活化实现 PFOS 的高效降解。转移的电子和产生的羟基自由基 (•OH) 可以高效分解 PFOS。电子可以通过 D-Ce/Sb 2 O快速从 PFOS 的最高占据分子轨道转移到 PMS 的最低未占据分子轨道3由电化学过程下的势能差驱动。D-Ce/Sb 2 O 3中Ce-O的活性位点可以大大缩短电子与•OH的迁移距离,从而提高降解各种有机微污染物的高稳定性催化活性。此外,电化学过程对变化的pH值、无机离子和有机物表现出很强的抵抗力和耐受性。这项研究提供了对通过电化学氧化去除 PFOS 的电子转移途径和 PMS 活化机制的见解,为其在水净化中的潜在应用铺平了道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号