Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2022-06-17 , DOI: 10.1016/j.jhazmat.2022.129407 Ying Wang 1 , Zaiwen Lin 1 , Jiahui Zhu 1 , Jingyuan Liu 1 , Jing Yu 1 , Qi Liu 2 , Rongrong Chen 3 , Ying Li 4 , Jun Wang 3
|
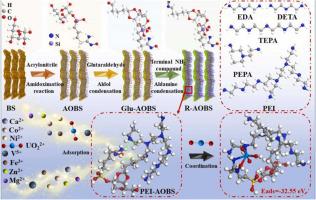
Efficiently capturing of uranium (VI) [U(VI)] from seawater elicits unparalleled attraction for sustaining the uplifted requirement for nuclear fuel. However, obtaining the abundant U(VI) resource from seawater has always seriously restricted by competitive adsorption from higher concentrations of competitors, especially vanadium (V) [V(V)]. Herein, based on amidoximized natural bamboo strips with hierarchical porous structure, the molecular-level uranyl-specific “nano-holes” was co-constructed by the intramolecular hydrogen bonds for specifically trapping U(VI) from seawater. Manipulating the branched degrees of amino groups enabled the creation of a series of the molecular-level uranyl-specific “nano-holes” that exhibit ultrahigh affinity and selective adsorption of U(VI) with a adsorption capacity 1.8 fold higher compared to that of V(V) after 30 days floating in the Yellow Sea basin, conquering the long-term challenge of the competitive adsorption of V(V) for amidoxime-based adsorbents applied to extract U(VI) from seawater. The diameter of the molecular-level uranyl-specific “nano-holes” is approximately 12.07 Å, significantly larger than (UO2)3(OH)3+ (10.37 Å) and smaller than HV10O285-, thereby exhibiting specifically trapping of U(VI) in a series of adsorption experiments with different U(VI)-V(V) ratios. Besides, the adsorption model based on the combination of experimental and theoretical results is accompanied by "hydrogen bond breaking and coordination bond formation".
中文翻译:

在天然竹条上与偕胺肟和氨基共构分子水平铀特异性“纳米孔”,用于从海水中特异性捕获铀
从海水中有效捕获铀 (VI) [U(VI)] 对维持对核燃料的高需求具有无与伦比的吸引力。然而,从海水中获取丰富的 U(VI) 资源一直受到来自较高浓度竞争对手的竞争吸附的严重限制,特别是钒 (V) [V(V)]。在此,基于具有分级多孔结构的偕胺肟化天然竹条,通过分子内氢键共构建分子水平铀酰特异性“纳米孔”,用于从海水中特异性捕获U(VI)。操纵氨基的支化度能够产生一系列分子水平的铀酰特异性“纳米孔”,这些“纳米孔”表现出对 U(VI) 的超高亲和力和选择性吸附,吸附能力为 1。在黄海盆地漂浮 30 天后,与 V(V) 相比高 8 倍,克服了用于从海水中提取 U(VI) 的偕胺肟基吸附剂竞争吸附 V(V) 的长期挑战. 分子级铀酰特异性“纳米孔”的直径约为 12.07 Å,明显大于(UO2 ) 3 (OH) 3 + (10.37 Å) 且小于 HV 10 O 28 5-,从而在一系列具有不同 U(VI)-V(V) 比率的吸附实验中表现出对 U(VI) 的特异性捕获。此外,基于实验和理论结果相结合的吸附模型伴随着“氢键断裂和配位键形成”。









































 京公网安备 11010802027423号
京公网安备 11010802027423号