Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electroprecipitation of Nanometer-Thick Films of Ln(OH)3 [Ln = La, Ce, and Lu] at Pt Microelectrodes and Their Effect on Electron-Transfer Reactions
Langmuir ( IF 3.7 ) Pub Date : 2022-06-17 , DOI: 10.1021/acs.langmuir.2c01008 Pavel Majumdar 1 , Rui Gao 1 , Henry S White 1
Langmuir ( IF 3.7 ) Pub Date : 2022-06-17 , DOI: 10.1021/acs.langmuir.2c01008 Pavel Majumdar 1 , Rui Gao 1 , Henry S White 1
Affiliation
|
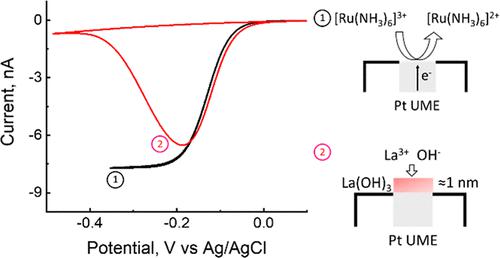
We report investigations of the deposition of nanometer-thick Ln(OH)3 films (Ln = La, Ce, and Lu) and their effect on outer-sphere and inner-sphere electron-transfer reactions. Insoluble Ln(OH)3 films are deposited from aqueous solutions of LaCl3 onto the surface of 12.5 μm radius Pt microdisk electrodes during water or oxygen reduction. Both reactions produce interfacial OH–, which complexes with Ln3+, resulting in the precipitation of Ln(OH)3. Surface analyses by scanning electron microscopy (SEM), SEM–energy-dispersive X-ray spectroscopy, and atomic force microscopy indicate the formation of a 1–2 nm thick uniform film. Outer-sphere electron-transfer reactions (Ru(NH3)63+ reduction, FcMeOH oxidation, and Fe(CN)64–/3– oxidation/reduction) were investigated at Ln(OH)3-modified electrodes of different film thicknesses. The results demonstrate that the steady-state transport-limited current for these reactions decreases with an increase in the film thickness. Moreover, the degree of blockage depends upon the redox species, suggesting that the Ln(OH)3 films are free from pinholes greater than the size of the redox molecules. This suggests that the films are either ionically conducting or that electron tunneling occurs across these thin layers. A similar blocking effect was observed for the inner-sphere reductions of H2O and O2. We further demonstrate that the thickness of La(OH)3 films can be controlled by anodic dissolution. Additionally, we show that La3+ lowers the supersaturation of dissolved H2 required to nucleate a stable nanobubble.
中文翻译:

在 Pt 微电极上的 Ln(OH)3 [Ln = La、Ce 和 Lu] 纳米厚膜的电沉淀及其对电子转移反应的影响
我们报告了对纳米厚 Ln(OH) 3薄膜(Ln = La、Ce 和 Lu)的沉积及其对外球和内球电子转移反应的影响的研究。在水或氧还原过程中,不溶性 Ln(OH) 3薄膜从 LaCl 3水溶液沉积到半径为 12.5 μm 的 Pt 微盘电极的表面上。两种反应都产生界面 OH - ,它与 Ln 3+络合,导致 Ln(OH) 3沉淀。通过扫描电子显微镜 (SEM)、SEM 能量色散 X 射线光谱和原子力显微镜进行的表面分析表明形成了 1-2 nm 厚的均匀薄膜。外层电子转移反应(Ru(NH3 )在不同膜厚的 Ln(OH) 3改性电极上研究了6 3+还原、FcMeOH 氧化和 Fe(CN) 6 4-/3-氧化/还原)。结果表明,这些反应的稳态传输限制电流随着膜厚度的增加而降低。此外,阻塞程度取决于氧化还原物质,这表明 Ln(OH) 3膜没有大于氧化还原分子大小的针孔。这表明这些薄膜要么是离子导电的,要么是在这些薄层上发生电子隧穿。对于 H 2 O 和 O的内球还原,观察到类似的阻塞效应2 . 我们进一步证明了La(OH) 3薄膜的厚度可以通过阳极溶解来控制。此外,我们表明 La 3+降低了使稳定的纳米气泡成核所需的溶解 H 2的过饱和度。
更新日期:2022-06-17
中文翻译:

在 Pt 微电极上的 Ln(OH)3 [Ln = La、Ce 和 Lu] 纳米厚膜的电沉淀及其对电子转移反应的影响
我们报告了对纳米厚 Ln(OH) 3薄膜(Ln = La、Ce 和 Lu)的沉积及其对外球和内球电子转移反应的影响的研究。在水或氧还原过程中,不溶性 Ln(OH) 3薄膜从 LaCl 3水溶液沉积到半径为 12.5 μm 的 Pt 微盘电极的表面上。两种反应都产生界面 OH - ,它与 Ln 3+络合,导致 Ln(OH) 3沉淀。通过扫描电子显微镜 (SEM)、SEM 能量色散 X 射线光谱和原子力显微镜进行的表面分析表明形成了 1-2 nm 厚的均匀薄膜。外层电子转移反应(Ru(NH3 )在不同膜厚的 Ln(OH) 3改性电极上研究了6 3+还原、FcMeOH 氧化和 Fe(CN) 6 4-/3-氧化/还原)。结果表明,这些反应的稳态传输限制电流随着膜厚度的增加而降低。此外,阻塞程度取决于氧化还原物质,这表明 Ln(OH) 3膜没有大于氧化还原分子大小的针孔。这表明这些薄膜要么是离子导电的,要么是在这些薄层上发生电子隧穿。对于 H 2 O 和 O的内球还原,观察到类似的阻塞效应2 . 我们进一步证明了La(OH) 3薄膜的厚度可以通过阳极溶解来控制。此外,我们表明 La 3+降低了使稳定的纳米气泡成核所需的溶解 H 2的过饱和度。









































 京公网安备 11010802027423号
京公网安备 11010802027423号