Structure ( IF 4.4 ) Pub Date : 2022-06-17 , DOI: 10.1016/j.str.2022.05.017 Katherine H Reiter 1 , Alex Zelter 1 , Maria K Janowska 1 , Michael Riffle 1 , Nicholas Shulman 2 , Brendan X MacLean 2 , Kaipo Tamura 2 , Matthew C Chambers 2 , Michael J MacCoss 2 , Trisha N Davis 1 , Miklos Guttman 3 , Peter S Brzovic 1 , Rachel E Klevit 1
|
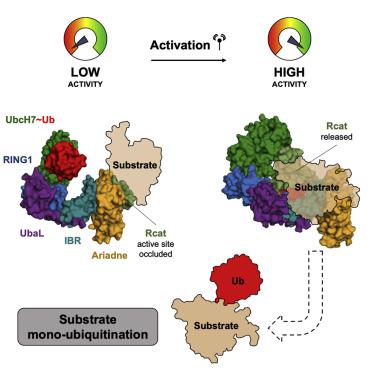
RING-between-RING (RBR) E3 ligases mediate ubiquitin transfer through an obligate E3-ubiquitin thioester intermediate prior to substrate ubiquitination. Although RBRs share a conserved catalytic module, substrate recruitment mechanisms remain enigmatic, and the relevant domains have yet to be identified for any member of the class. Here we characterize the interaction between the auto-inhibited RBR, HHARI (AriH1), and its target protein, 4EHP, using a combination of XL-MS, HDX-MS, NMR, and biochemical studies. The results show that (1) a di-aromatic surface on the catalytic HHARI Rcat domain forms a binding platform for substrates and (2) a phosphomimetic mutation on the auto-inhibitory Ariadne domain of HHARI promotes release and reorientation of Rcat for transthiolation and substrate modification. The findings identify a direct binding interaction between a RING-between-RING ligase and its substrate and suggest a general model for RBR substrate recognition.
中文翻译:

动态 RBR 催化结构域对 HHARI 底物的 Cullin 独立识别
环间环 (RBR) E3 连接酶在底物泛素化之前通过专性 E3-泛素硫酯中间体介导泛素转移。尽管 RBR 共享一个保守的催化模块,但底物招募机制仍然是个谜,并且尚未为该类的任何成员确定相关域。在这里,我们结合 XL-MS、HDX-MS、NMR 和生化研究来表征自动抑制 RBR、HHARI (AriH1) 及其靶蛋白 4EHP 之间的相互作用。结果表明,(1) 催化 HHARI Rcat 结构域上的双芳香族表面形成底物的结合平台,(2) HHARI 的自抑制 Ariadne 结构域上的磷模拟突变促进 Rcat 的释放和重新定向,以进行转硫基作用和底物修改。这些发现确定了环间连接酶与其底物之间的直接结合相互作用,并提出了 RBR 底物识别的通用模型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号