当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gold(I)-Catalyzed Selective Hydroarylation of Indoles with Haloalkynes
Organic Letters ( IF 4.9 ) Pub Date : 2022-06-17 , DOI: 10.1021/acs.orglett.2c01921 Cunbo Wei 1 , Jiawen Wu 1 , Lizhu Zhang 1 , Zhonghua Xia 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-06-17 , DOI: 10.1021/acs.orglett.2c01921 Cunbo Wei 1 , Jiawen Wu 1 , Lizhu Zhang 1 , Zhonghua Xia 1
Affiliation
|
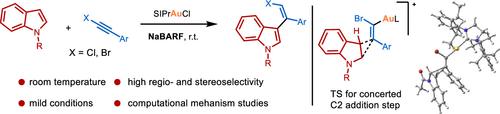
A highly regio- and stereoselective synthesis of a Z-alkenyl indole via the gold-catalyzed addition of an indole to a haloalkyne was developed. In the presence of gold catalyst SIPrAuCl and cocatalyst NaBARF, a broad range of indoles react with haloalkynes to afford Z-alkenyl indoles with high selectivity at room temperature. Computational studies suggest that the hydroarylation reaction takes place via a concerted C2 addition pathway of the indole to the activated haloalkyne.
中文翻译:

金(I)催化的卤代炔烃选择性氢化吲哚
开发了通过金催化将吲哚加成到卤代炔烃上的Z-烯基吲哚的高度区域和立体选择性合成。在金催化剂 SIPrAuCl 和助催化剂 NaBARF 的存在下,多种吲哚与卤代炔烃反应生成Z-烯基吲哚,在室温下具有高选择性。计算研究表明,氢化芳基化反应通过吲哚与活化卤代炔烃的协同 C2 加成途径发生。
更新日期:2022-06-17
中文翻译:

金(I)催化的卤代炔烃选择性氢化吲哚
开发了通过金催化将吲哚加成到卤代炔烃上的Z-烯基吲哚的高度区域和立体选择性合成。在金催化剂 SIPrAuCl 和助催化剂 NaBARF 的存在下,多种吲哚与卤代炔烃反应生成Z-烯基吲哚,在室温下具有高选择性。计算研究表明,氢化芳基化反应通过吲哚与活化卤代炔烃的协同 C2 加成途径发生。










































 京公网安备 11010802027423号
京公网安备 11010802027423号