Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Carbon-Based Biosensing Platform for Simultaneously Measuring the Contraction and Electrophysiology of iPSC-Cardiomyocyte Monolayers
ACS Nano ( IF 15.8 ) Pub Date : 2022-06-17 , DOI: 10.1021/acsnano.2c04676 Wenkun Dou 1, 2 , Manpreet Malhi 2, 3 , Teng Cui 1 , Minyao Wang 4, 5 , Tiancong Wang 1 , Guanqiao Shan 1 , Junhui Law 1 , Zheyuan Gong 1 , Julia Plakhotnik 2, 3 , Tobin Filleter 1 , Renke Li 5 , Craig A Simmons 1, 4, 6 , Jason T Maynes 2, 3, 7 , Yu Sun 1, 4, 8, 9
ACS Nano ( IF 15.8 ) Pub Date : 2022-06-17 , DOI: 10.1021/acsnano.2c04676 Wenkun Dou 1, 2 , Manpreet Malhi 2, 3 , Teng Cui 1 , Minyao Wang 4, 5 , Tiancong Wang 1 , Guanqiao Shan 1 , Junhui Law 1 , Zheyuan Gong 1 , Julia Plakhotnik 2, 3 , Tobin Filleter 1 , Renke Li 5 , Craig A Simmons 1, 4, 6 , Jason T Maynes 2, 3, 7 , Yu Sun 1, 4, 8, 9
Affiliation
|
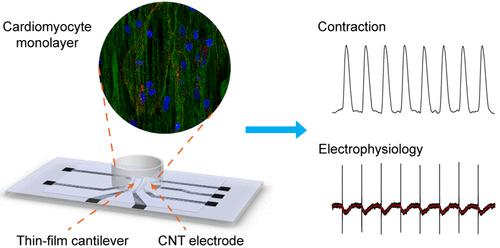
Heart beating is triggered by the generation and propagation of action potentials through the myocardium, resulting in the synchronous contraction of cardiomyocytes. This process highlights the importance of electrical and mechanical coordination in organ function. Investigating the pathogenesis of heart diseases and potential therapeutic actions in vitro requires biosensing technologies which allow for long-term and simultaneous measurement of the contractility and electrophysiology of cardiomyocytes. However, the adoption of current biosensing approaches for functional measurement of in vitro cardiac models is hampered by low sensitivity, difficulties in achieving multifunctional detection, and costly manufacturing processes. Leveraging carbon-based nanomaterials, we developed a biosensing platform that is capable of performing on-chip and simultaneous measurement of contractility and electrophysiology of human induced pluripotent stem-cell-derived cardiomyocyte (iPSC-CM) monolayers. This platform integrates with a flexible thin-film cantilever embedded with a carbon black (CB)-PDMS strain sensor for high-sensitivity contraction measurement and four pure carbon nanotube (CNT) electrodes for the detection of extracellular field potentials with low electrode impedance. Cardiac functional properties including contractile stress, beating rate, beating rhythm, and extracellular field potential were evaluated to quantify iPSC-CM responses to common cardiotropic agents. In addition, an in vitro model of drug-induced cardiac arrhythmia was established to further validate the platform for disease modeling and drug testing.
中文翻译:

用于同时测量 iPSC 心肌细胞单层收缩和电生理学的碳基生物传感平台
心脏跳动是由动作电位的产生和传播通过心肌引起的,导致心肌细胞同步收缩。这个过程突出了机电协调在器官功能中的重要性。研究心脏病的发病机制和潜在的体外治疗作用需要生物传感技术,这种技术可以长期同时测量心肌细胞的收缩力和电生理学。然而,采用当前的生物传感方法进行体外功能测量心脏模型受到低灵敏度、难以实现多功能检测以及昂贵的制造过程的阻碍。利用碳基纳米材料,我们开发了一个生物传感平台,能够在芯片上同时测量人诱导多能干细胞衍生心肌细胞 (iPSC-CM) 单层细胞的收缩性和电生理学。该平台集成了一个柔性薄膜悬臂,该悬臂嵌入了用于高灵敏度收缩测量的炭黑 (CB)-PDMS 应变传感器,以及用于检测具有低电极阻抗的细胞外场电位的四个纯碳纳米管 (CNT) 电极。心脏功能特性,包括收缩应力、搏动率、搏动节律、和细胞外场电位进行评估,以量化 iPSC-CM 对常见心脏药物的反应。此外,一个建立药物性心律失常体外模型,进一步验证疾病建模和药物测试平台。
更新日期:2022-06-17
中文翻译:

用于同时测量 iPSC 心肌细胞单层收缩和电生理学的碳基生物传感平台
心脏跳动是由动作电位的产生和传播通过心肌引起的,导致心肌细胞同步收缩。这个过程突出了机电协调在器官功能中的重要性。研究心脏病的发病机制和潜在的体外治疗作用需要生物传感技术,这种技术可以长期同时测量心肌细胞的收缩力和电生理学。然而,采用当前的生物传感方法进行体外功能测量心脏模型受到低灵敏度、难以实现多功能检测以及昂贵的制造过程的阻碍。利用碳基纳米材料,我们开发了一个生物传感平台,能够在芯片上同时测量人诱导多能干细胞衍生心肌细胞 (iPSC-CM) 单层细胞的收缩性和电生理学。该平台集成了一个柔性薄膜悬臂,该悬臂嵌入了用于高灵敏度收缩测量的炭黑 (CB)-PDMS 应变传感器,以及用于检测具有低电极阻抗的细胞外场电位的四个纯碳纳米管 (CNT) 电极。心脏功能特性,包括收缩应力、搏动率、搏动节律、和细胞外场电位进行评估,以量化 iPSC-CM 对常见心脏药物的反应。此外,一个建立药物性心律失常体外模型,进一步验证疾病建模和药物测试平台。











































 京公网安备 11010802027423号
京公网安备 11010802027423号