当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of Redox-Promoted Brønsted Acid Catalysis in the Gold(III)-Catalyzed Annulation of Phenol and Cyclohexadiene
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-06-17 , DOI: 10.1021/acscatal.2c01194 Kaveh Farshadfar 1 , Andrew J. Tague 2 , Mohammad Talebi 3 , Brian F. Yates 3 , Christopher J. T. Hyland 2 , Alireza Ariafard 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-06-17 , DOI: 10.1021/acscatal.2c01194 Kaveh Farshadfar 1 , Andrew J. Tague 2 , Mohammad Talebi 3 , Brian F. Yates 3 , Christopher J. T. Hyland 2 , Alireza Ariafard 3
Affiliation
|
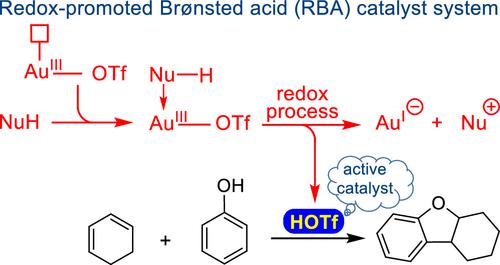
This study discovers a mechanism called redox-promoted Brønsted acid activation using DFT calculations through mechanistic elucidation of the phenol and cyclohexadiene annulation catalyzed by the AuCl3/AgOTf mixed system. According to this mechanism, triflic acid (HOTf) is likely to be the active catalyst generated in situ as a result of the irreversible reduction of gold(III) to gold(I). The corresponding annulation reaction proceeds through two linked catalytic cycles, the first of which conducts the hydroarylation of diene with phenol and is significantly faster than the second, which produces the observed product via intramolecular cyclization. The [OTf]− counteranion of HOTf is found to play an important role in preventing the polymerization of cyclohexadiene. To confirm that HOTf is the active catalyst in both catalytic cycles of the annulation process, we performed experiments with HOTf as the catalyst and achieved the same product as when AuCl3/AgOTf was used as the catalyst. Additionally, NMR spectroscopy and ESI-MS experiments supported the production of the Au(I) ion and HOTf Brønsted acid.
中文翻译:

在金(III)催化的苯酚和环己二烯环化中发现氧化还原促进的布朗斯台德酸催化
本研究通过对 AuCl 3 /AgOTf 混合体系催化的苯酚和环己二烯环化的机理阐明,利用 DFT 计算发现了一种称为氧化还原促进布朗斯台德酸活化的机制。根据这一机制,三氟甲磺酸(HOTf)很可能是由于金(III)不可逆地还原为金(I)而原位产生的活性催化剂。相应的环化反应通过两个连接的催化循环进行,第一个催化循环进行二烯与苯酚的加氢芳基化反应,并且明显快于第二个催化循环,后者通过分子内环化产生观察到的产物。[OTf] -发现 HOTf 的抗衡阴离子在防止环己二烯聚合中起重要作用。为了证实 HOTf 在环化过程的两个催化循环中都是活性催化剂,我们使用 HOTf 作为催化剂进行了实验,并获得了与使用 AuCl 3 /AgOTf 作为催化剂时相同的产物。此外,NMR 光谱和 ESI-MS 实验支持了 Au(I) 离子和 HOTf 布朗斯台德酸的产生。
更新日期:2022-06-17
中文翻译:

在金(III)催化的苯酚和环己二烯环化中发现氧化还原促进的布朗斯台德酸催化
本研究通过对 AuCl 3 /AgOTf 混合体系催化的苯酚和环己二烯环化的机理阐明,利用 DFT 计算发现了一种称为氧化还原促进布朗斯台德酸活化的机制。根据这一机制,三氟甲磺酸(HOTf)很可能是由于金(III)不可逆地还原为金(I)而原位产生的活性催化剂。相应的环化反应通过两个连接的催化循环进行,第一个催化循环进行二烯与苯酚的加氢芳基化反应,并且明显快于第二个催化循环,后者通过分子内环化产生观察到的产物。[OTf] -发现 HOTf 的抗衡阴离子在防止环己二烯聚合中起重要作用。为了证实 HOTf 在环化过程的两个催化循环中都是活性催化剂,我们使用 HOTf 作为催化剂进行了实验,并获得了与使用 AuCl 3 /AgOTf 作为催化剂时相同的产物。此外,NMR 光谱和 ESI-MS 实验支持了 Au(I) 离子和 HOTf 布朗斯台德酸的产生。











































 京公网安备 11010802027423号
京公网安备 11010802027423号