当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insights into the Atomistic Mechanisms of Phosphorylation in Disrupting Liquid–Liquid Phase Separation and Aggregation of the FUS Low-Complexity Domain
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-06-16 , DOI: 10.1021/acs.jcim.2c00414 Zenghui Lao 1 , Xuewei Dong 1 , Xianshi Liu 1 , Fangying Li 1 , Yujie Chen 1 , Yiming Tang 1 , Guanghong Wei 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-06-16 , DOI: 10.1021/acs.jcim.2c00414 Zenghui Lao 1 , Xuewei Dong 1 , Xianshi Liu 1 , Fangying Li 1 , Yujie Chen 1 , Yiming Tang 1 , Guanghong Wei 1
Affiliation
|
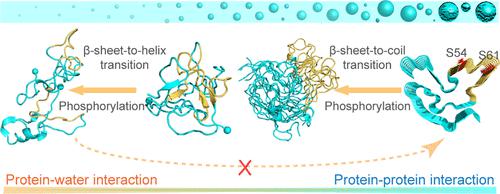
Fused in sarcoma (FUS), a nuclear RNA binding protein, can not only undergo liquid–liquid phase separation (LLPS) to form dynamic biomolecular condensates but also aggregate into solid amyloid fibrils which are associated with the pathology of amyotrophic lateral sclerosis and frontotemporal lobar degeneration diseases. Phosphorylation in the FUS low-complexity domain (FUS-LC) inhibits FUS LLPS and aggregation. However, it remains largely elusive what are the underlying atomistic mechanisms of this inhibitory effect and whether phosphorylation can disrupt preformed FUS fibrils, reversing the FUS gel/solid phase toward the liquid phase. Herein, we systematically investigate the impacts of phosphorylation on the conformational ensemble of the FUS37–97 monomer and dimer and the structure of the FUS37–97 fibril by performing extensive all-atom molecular dynamics simulations. Our simulations reveal three key findings: (1) phosphorylation shifts the conformations of FUS37–97 from the β-rich, fibril-competent state toward a helix-rich, fibril-incompetent state; (2) phosphorylation significantly weakens protein–protein interactions and enhances protein–water interactions, which disfavor FUS-LC LLPS as well as aggregation and facilitate the dissolution of the preformed FUS-LC fibril; and (3) the FUS37–97 peptide displays a high β-strand probability in the region spanning residues 52–67, and phosphorylation at S54 and S61 residues located in this region is crucial for the disruption of LLPS and aggregation of FUS-LC. This study may pave the way for ameliorating phase-separation-related pathologies via site-specific phosphorylation.
中文翻译:

深入了解磷酸化在破坏 FUS 低复杂度域的液-液相分离和聚集中的原子机制
融合肉瘤 (FUS) 是一种核 RNA 结合蛋白,不仅可以通过液-液相分离 (LLPS) 形成动态生物分子凝聚物,而且可以聚集成与肌萎缩侧索硬化和额颞叶病理相关的固体淀粉样蛋白原纤维退化性疾病。FUS 低复杂性结构域 (FUS-LC) 中的磷酸化抑制 FUS LLPS 和聚集。然而,这种抑制作用的潜在原子机制是什么以及磷酸化是否可以破坏预先形成的 FUS 原纤维,将 FUS 凝胶/固相逆转为液相,这在很大程度上仍然难以捉摸。在这里,我们系统地研究了磷酸化对 FUS 37-97单体和二聚体的构象集合以及 FUS 结构的影响。37-97原纤维通过进行广泛的全原子分子动力学模拟。我们的模拟揭示了三个关键发现:(1)磷酸化将 FUS 37-97的构象从富含 β、原纤维的状态转变为富含螺旋、原纤维的状态;(2) 磷酸化显着削弱蛋白质-蛋白质相互作用并增强蛋白质-水相互作用,这不利于 FUS-LC LLPS 以及聚集并促进预制 FUS-LC 原纤维的溶解;(3) FUS 37-97肽在跨越残基 52-67 的区域中显示出高 β 链概率,位于该区域的 S54 和 S61 残基的磷酸化对于 LLPS 的破坏和 FUS-LC 的聚集至关重要。该研究可能为通过位点特异性磷酸化改善相分离相关病理铺平道路。
更新日期:2022-06-16
中文翻译:

深入了解磷酸化在破坏 FUS 低复杂度域的液-液相分离和聚集中的原子机制
融合肉瘤 (FUS) 是一种核 RNA 结合蛋白,不仅可以通过液-液相分离 (LLPS) 形成动态生物分子凝聚物,而且可以聚集成与肌萎缩侧索硬化和额颞叶病理相关的固体淀粉样蛋白原纤维退化性疾病。FUS 低复杂性结构域 (FUS-LC) 中的磷酸化抑制 FUS LLPS 和聚集。然而,这种抑制作用的潜在原子机制是什么以及磷酸化是否可以破坏预先形成的 FUS 原纤维,将 FUS 凝胶/固相逆转为液相,这在很大程度上仍然难以捉摸。在这里,我们系统地研究了磷酸化对 FUS 37-97单体和二聚体的构象集合以及 FUS 结构的影响。37-97原纤维通过进行广泛的全原子分子动力学模拟。我们的模拟揭示了三个关键发现:(1)磷酸化将 FUS 37-97的构象从富含 β、原纤维的状态转变为富含螺旋、原纤维的状态;(2) 磷酸化显着削弱蛋白质-蛋白质相互作用并增强蛋白质-水相互作用,这不利于 FUS-LC LLPS 以及聚集并促进预制 FUS-LC 原纤维的溶解;(3) FUS 37-97肽在跨越残基 52-67 的区域中显示出高 β 链概率,位于该区域的 S54 和 S61 残基的磷酸化对于 LLPS 的破坏和 FUS-LC 的聚集至关重要。该研究可能为通过位点特异性磷酸化改善相分离相关病理铺平道路。



























 京公网安备 11010802027423号
京公网安备 11010802027423号