当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper-Catalyzed 6-endo-dig Cyclization–Coupling of 2-Bromoaryl Ketones and Terminal Alkynes toward Naphthyl Aryl Ethers in Water
Organic Letters ( IF 4.9 ) Pub Date : 2022-06-17 , DOI: 10.1021/acs.orglett.2c01654 Lebin Su 1, 2 , Shimin Xie 1 , Jianyu Dong 2 , Neng Pan 1 , Shuang-Feng Yin 1 , Yongbo Zhou 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-06-17 , DOI: 10.1021/acs.orglett.2c01654 Lebin Su 1, 2 , Shimin Xie 1 , Jianyu Dong 2 , Neng Pan 1 , Shuang-Feng Yin 1 , Yongbo Zhou 1
Affiliation
|
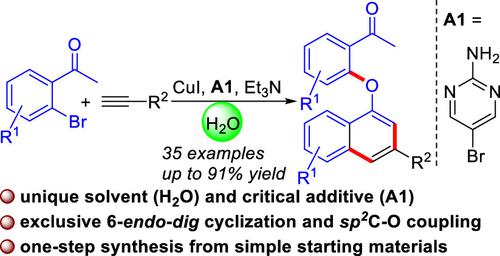
The cyclization–coupling reaction of 2-bromoaryl ketones and terminal alkynes is first realized by copper catalysis, which produces polyfunctional naphthyl aryl ethers in moderate to excellent yields with broad substrate scope and good functional group tolerance. This reaction proceeds via 6-endo-dig cyclization and C(sp2)–O coupling using green H2O as the unique solvent and 5-bromopyrimidin-2-amine as the critical additive. Mechanistically, a unique Cu(III)-acetylide probably is the key intermediate, which allows exclusive 6-endo-dig selectivity.
中文翻译:

铜催化的 6-endo-dig 环化——2-溴芳基酮和末端炔烃在水中与萘基芳基醚的偶联
2-溴芳基酮和末端炔烃的环化偶联反应首先通过铜催化实现,该反应以中等至优异的收率产生多官能萘基芳基醚,具有广泛的底物范围和良好的官能团耐受性。该反应通过 6 - endo - dig环化和 C(sp 2 )-O 偶联进行,使用绿色 H 2 O 作为唯一溶剂,5-溴嘧啶-2-胺作为关键添加剂。从机理上讲,独特的 Cu(III)-乙炔化物可能是关键的中间体,它允许独有的 6 - endo - dig选择性。
更新日期:2022-06-17
中文翻译:

铜催化的 6-endo-dig 环化——2-溴芳基酮和末端炔烃在水中与萘基芳基醚的偶联
2-溴芳基酮和末端炔烃的环化偶联反应首先通过铜催化实现,该反应以中等至优异的收率产生多官能萘基芳基醚,具有广泛的底物范围和良好的官能团耐受性。该反应通过 6 - endo - dig环化和 C(sp 2 )-O 偶联进行,使用绿色 H 2 O 作为唯一溶剂,5-溴嘧啶-2-胺作为关键添加剂。从机理上讲,独特的 Cu(III)-乙炔化物可能是关键的中间体,它允许独有的 6 - endo - dig选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号