Phytomedicine ( IF 6.7 ) Pub Date : 2022-06-15 , DOI: 10.1016/j.phymed.2022.154241 Yan-Fang Deng 1 , Qian-Qian Xu 1 , Tian-Qi Chen 2 , Jia-Xiong Ming 1 , Ya-Fen Wang 1 , Li-Na Mao 1 , Jia-Jun Zhou 1 , Wei-Guang Sun 1 , Qun Zhou 1 , Hong Ren 3 , Yong-Hui Zhang 1
|
Background
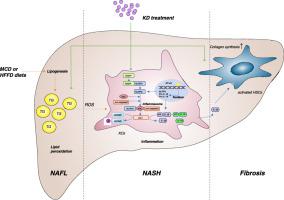
Non-alcoholic steatohepatitis (NASH) has replaced viral hepatitis as the main driver of the rising morbidity and mortality associated with cirrhosis and liver cancer worldwide, while no FDA-approved therapies are currently known. Kinsenoside (KD), naturally isolated from Anoectochilus roxburghii, possesses multiple biological activities, including lipolysis, anti-inflammation, and hepatoprotection. However, the effects of KD on NASH remain unclear.
Purpose
This study aimed to explore the roles of KD in NASH and its engaged mechanisms.
Methods
Two typical animal models of NASH, mice fed a methionine-choline-deficient (MCD) diet (representing non-obese NASH) and mice fed a high-fat and -fructose diet (HFFD) (representing obese NASH), were used to investigate the effect of KD on NASH in vivo. Transcriptome sequencing was performed to elucidate the underlying mechanisms of KD. Lipopolysaccharide (LPS)-stimulated THP-1 cells and transforming growth factor β1 (TGF-β1)-activated LX-2 cells were applied to further explore the effects and mechanisms of KD in vitro.
Results
The intragastric administration of KD remarkably alleviated MCD/HFFD-induced murine NASH almost in a dose-dependent manner. Specifically, KD reduced lipid accumulation, inflammation, and fibrosis in the liver of NASH mice. KD ameliorated alanine aminotransferase (ALT), aspartate aminotransferase (AST), superoxide dismutase (SOD), and malondialdehyde (MDA) abnormalities. In addition, it decreased the level of serum proinflammatory factors (IL-12p70, IL-6, TNF-α, MCP-1, IFN-γ) and the hepatic expression of typical fibrosis-related molecules (α-SMA, Col-I, TIMP-1). Mechanically, KD attenuated the MCD/HFFD-induced NASH through the inhibition of the NF-κB/NLRP3 signaling pathway. Consistently, KD reduced inflammation stimulated by LPS in THP-1 cells via suppressing the NF-κB/NLRP3 pathway. Furthermore, it prevented the activation of LX-2 cells directly, by inhibiting the proliferation stimulated by TGF-β1, and indirectly, by inactivating the NLRP3 inflammasome in macrophages.
Conclusion
For the first time, the practical improvement of NASH by KD was revealed. Our study found that KD exerted its alleviative effects on NASH through the inhibition of the NF-κB/NLRP3 signaling pathway. Given its hepatoprotective and nontoxic properties, KD has the potential to be a novel and effective drug to treat NASH.
中文翻译:

Kinsenoside 通过抑制 NF-κB/NLRP3 信号通路减轻实验性 NASH 小鼠的炎症和纤维化
背景
非酒精性脂肪性肝炎 (NASH) 已取代病毒性肝炎,成为全球肝硬化和肝癌相关发病率和死亡率上升的主要驱动因素,而目前尚无 FDA 批准的治疗方法。Kinsenoside (KD) 是从金线莲中天然分离出来的,具有多种生物活性,包括脂肪分解、抗炎和保肝作用。然而,KD 对 NASH 的影响仍不清楚。
目的
本研究旨在探讨 KD 在 NASH 中的作用及其参与机制。
方法
两种典型的 NASH 动物模型,喂食蛋氨酸-胆碱缺乏 (MCD) 饮食的小鼠(代表非肥胖 NASH)和喂食高脂肪和果糖饮食 (HFFD) 的小鼠(代表肥胖 NASH),用于研究KD对体内NASH的影响。进行转录组测序以阐明 KD 的潜在机制。应用脂多糖 (LPS) 刺激的 THP-1 细胞和转化生长因子 β1 (TGF-β1) 激活的 LX-2 细胞进一步探索体外KD 的作用和机制。
结果
KD 的胃内给药几乎以剂量依赖性方式显着减轻了 MCD/HFFD 诱导的小鼠 NASH。具体来说,KD 减少了 NASH 小鼠肝脏中的脂质积累、炎症和纤维化。KD 改善了丙氨酸氨基转移酶 (ALT)、天冬氨酸氨基转移酶 (AST)、超氧化物歧化酶 (SOD) 和丙二醛 (MDA) 异常。此外,它还降低了血清促炎因子(IL-12p70、IL-6、TNF-α、MCP-1、IFN-γ)的水平和典型纤维化相关分子(α-SMA、Col-I)的肝脏表达。 , TIMP-1)。机械地,KD 通过抑制 NF-κB/NLRP3 信号通路减弱 MCD/HFFD 诱导的 NASH。与此一致,KD 通过抑制 NF-κB/NLRP3 通路减少了 THP-1 细胞中 LPS 刺激的炎症。此外,它直接阻止了 LX-2 细胞的活化,
结论
首次揭示了 KD 对 NASH 的实际改进。我们的研究发现,KD 通过抑制 NF-κB/NLRP3 信号通路发挥其对 NASH 的缓解作用。鉴于其保肝和无毒特性,KD 有可能成为治疗 NASH 的新型有效药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号