Minerals Engineering ( IF 4.9 ) Pub Date : 2022-06-16 , DOI: 10.1016/j.mineng.2022.107675 Wei Sung Ng , Yanhua Liu , Miao Chen
|
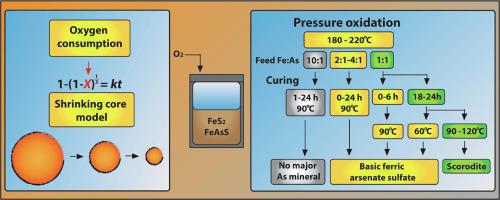
In the treatment of arsenic-bearing refractory gold ores, pressure oxidation (POX) represents an attractive approach to arsenic immobilisation, as the conditions are suitable for both sulfide oxidation and the formation of stable arsenates. While extensive work has been conducted on the reactions of the arsenic species during POX, research on the behaviour in the subsequent curing stage has been limited. The focus of this study is to explore the effect of curing time and temperature on the properties of the arsenic-bearing product following POX, using a synthetic mixture of pyrite and arsenopyrite.
Basic ferric arsenate sulfate (Fe(AsO4)1−x(SO4)x(OH)x.(1 − x)H2O) was observed as the major arsenic-bearing phase for Fe:As feeds of 1:1 and 2:1, and an increase in curing time resulted in higher arsenate stability based on characteristic leaching studies. A transformation to scorodite occurred for the 1:1 sample after 18–24 h of curing at 90–120 °C, along with a 60% increase in arsenic removal from solution and increased stability during characteristic leaching. Scorodite was absent at curing temperatures of 60 °C and for samples with a feed Fe:As above 2:1. At a feed Fe:As of 10:1, the major arsenic-bearing phases were amorphous iron arsenates, and arsenic concentrations in the leach extract were two orders of magnitude higher than 1:1 samples. Worryingly, this suggests that high arsenic-to-iron ratios are required for the formation of stable precipitates, with unstable phases forming otherwise.
Oxygen consumption data collected during POX was used to fit a shrinking core model for pyrite and arsenopyrite, and the reaction of pyrite in this work followed a mixed control of chemical and oxygen transport, while that of arsenopyrite was limited by surface chemical reaction. Pyrite oxidation was enhanced by the presence of arsenopyrite, likely due to the formation of Fe(III)-As(V) complexes that contribute to mineral oxidation, and the magnitude of the enhancement increased with temperature.
中文翻译:

硫化对砷析出的影响及黄铁矿和毒砂加压氧化动力学研究
在处理含砷难处理金矿石时,压力氧化 (POX) 代表了一种有吸引力的砷固定方法,因为该条件既适合硫化物氧化,又适合形成稳定的砷酸盐。虽然已经对 POX 过程中砷物质的反应进行了广泛的研究,但对后续固化阶段行为的研究却很有限。本研究的重点是使用黄铁矿和毒砂的合成混合物,探讨固化时间和温度对 POX 后含砷产品性能的影响。
碱性硫酸亚砷酸铁 (Fe(AsO 4 ) 1−x (SO 4 ) x (OH) x .(1 − x)H 2O) 被观察为 1:1 和 2:1 的 Fe:As 进料的主要含砷相,并且基于特征浸出研究,固化时间的增加导致砷酸盐稳定性更高。在 90-120 °C 下固化 18-24 小时后,1:1 样品发生了向臭葱石的转变,同时从溶液中去除砷的速度增加了 60%,并且在特征浸出过程中稳定性增加。在 60 °C 的固化温度和进料 Fe:As 高于 2:1 的样品中不存在 Scorodite。在进料 Fe:10:1 时,主要含砷相是无定形砷酸铁,浸出液中的砷浓度比 1:1 样品高两个数量级。令人担忧的是,这表明形成稳定的沉淀物需要高砷铁比,否则会形成不稳定的相。
POX 过程中收集的耗氧量数据用于拟合黄铁矿和毒砂的缩核模型,本工作中黄铁矿的反应遵循化学和氧气传输的混合控制,而毒砂的反应受表面化学反应的限制。毒砂的存在增强了黄铁矿的氧化,这可能是由于形成了有助于矿物氧化的 Fe(III)-As(V) 配合物,并且增强的幅度随着温度的升高而增加。











































 京公网安备 11010802027423号
京公网安备 11010802027423号