当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure, Diffusion, and Stability of Lithium Salts in Aprotic Dimethyl Sulfoxide and Acetonitrile Electrolytes
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2022-06-16 , DOI: 10.1021/acs.jpcc.2c02174 Zhen Jiang 1 , Andrew M. Rappe 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2022-06-16 , DOI: 10.1021/acs.jpcc.2c02174 Zhen Jiang 1 , Andrew M. Rappe 1
Affiliation
|
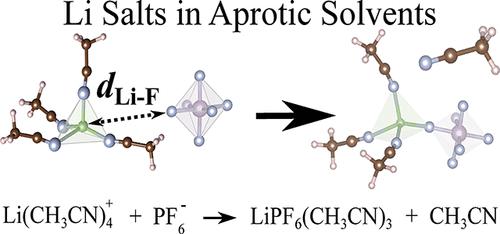
The development of lithium batteries, including lithium-ion batteries and lithium-air batteries, is the key technological breakthrough in the field of renewable energy storage. However, to date, it is still challenging to design a lithium battery with both high specific power and stability. Specifically, the solvation structure and thermodynamic stability of various electrolytes, mostly Li salts and aprotic solvents, have not been systematically studied from an atomic viewpoint. In this paper, we studied the solution chemistry of three most common inorganic Li salts (LiClO4, LiBF4, and LiPF6) and O2 in two aprotic (dimethyl sulfoxide (DMSO) and acetonitrile (CH3CN)) solvents. The Born–Oppenheimer molecular dynamics simulations and enhanced free energy samplings are employed to obtain their solvation structures, diffusion coefficients, and stability performances at room temperature. As a result, the tetrahedral Li(DMSO)4+ and Li(CH3CN)4+ are obtained as the stable solvation shells of Li+ in DMSO and CH3CN solvents, respectively. Among the three inorganic Li salts, the stability performances are found in the order of LiClO4 > LiBF4 > LiPF6 in both DMSO and CH3CN solvents. Compared with CH3CN, DMSO provides a more stable environment for the long-term usage of Li salts for that increases the energetic barriers of the degradation reactions of solvated Li+ components. However, DMSO shows a weaker ability (than CH3CN) to transport the main redox species (solvated Li+ and O2) in the electrolyte, which limits the discharging and charging rate in the batteries.
中文翻译:

非质子二甲基亚砜和乙腈电解质中锂盐的结构、扩散和稳定性
锂电池的发展,包括锂离子电池和锂空气电池,是可再生能源存储领域的关键技术突破。然而,迄今为止,设计兼具高比功率和稳定性的锂电池仍然具有挑战性。具体而言,尚未从原子角度系统地研究各种电解质(主要是锂盐和非质子溶剂)的溶剂化结构和热力学稳定性。在本文中,我们研究了三种最常见的无机锂盐(LiClO 4、LiBF 4和 LiPF 6)和 O 2在两种非质子(二甲基亚砜 (DMSO) 和乙腈 (CH 3CN)) 溶剂。Born-Oppenheimer 分子动力学模拟和增强的自由能采样用于获得它们在室温下的溶剂化结构、扩散系数和稳定性性能。结果,得到了四面体Li(DMSO) 4 +和Li(CH 3 CN) 4 +作为Li +在DMSO和CH 3 CN溶剂中的稳定溶剂化壳层。三种无机锂盐在DMSO和CH 3 CN溶剂中的稳定性能依次为LiClO 4 > LiBF 4 > LiPF 6 。与 CH 3相比CN、DMSO 为锂盐的长期使用提供了更稳定的环境,增加了溶剂化 Li +组分降解反应的能量屏障。然而,DMSO 在电解质中传输主要氧化还原物质(溶剂化 Li +和 O 2 )的能力(比 CH 3 CN 弱),这限制了电池的放电和充电速率。
更新日期:2022-06-16
中文翻译:

非质子二甲基亚砜和乙腈电解质中锂盐的结构、扩散和稳定性
锂电池的发展,包括锂离子电池和锂空气电池,是可再生能源存储领域的关键技术突破。然而,迄今为止,设计兼具高比功率和稳定性的锂电池仍然具有挑战性。具体而言,尚未从原子角度系统地研究各种电解质(主要是锂盐和非质子溶剂)的溶剂化结构和热力学稳定性。在本文中,我们研究了三种最常见的无机锂盐(LiClO 4、LiBF 4和 LiPF 6)和 O 2在两种非质子(二甲基亚砜 (DMSO) 和乙腈 (CH 3CN)) 溶剂。Born-Oppenheimer 分子动力学模拟和增强的自由能采样用于获得它们在室温下的溶剂化结构、扩散系数和稳定性性能。结果,得到了四面体Li(DMSO) 4 +和Li(CH 3 CN) 4 +作为Li +在DMSO和CH 3 CN溶剂中的稳定溶剂化壳层。三种无机锂盐在DMSO和CH 3 CN溶剂中的稳定性能依次为LiClO 4 > LiBF 4 > LiPF 6 。与 CH 3相比CN、DMSO 为锂盐的长期使用提供了更稳定的环境,增加了溶剂化 Li +组分降解反应的能量屏障。然而,DMSO 在电解质中传输主要氧化还原物质(溶剂化 Li +和 O 2 )的能力(比 CH 3 CN 弱),这限制了电池的放电和充电速率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号