当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into the Selectivity Determinant and Rate-Determining Step of CO2 Hydrogenation to Methanol
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2022-06-16 , DOI: 10.1021/acs.jpcc.2c02995 Chizhou Tang 1, 2 , Shan Tang 1, 2 , Feng Sha 1, 3 , Zhe Han 1, 3 , Zhendong Feng 1, 2 , Jijie Wang 1 , Can Li 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2022-06-16 , DOI: 10.1021/acs.jpcc.2c02995 Chizhou Tang 1, 2 , Shan Tang 1, 2 , Feng Sha 1, 3 , Zhe Han 1, 3 , Zhendong Feng 1, 2 , Jijie Wang 1 , Can Li 1
Affiliation
|
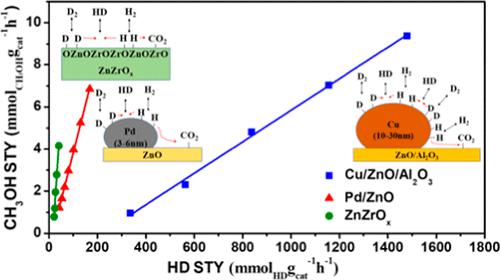
CO2 hydrogenation to methanol has attracted much attention. The mechanism, the factors affecting selectivity, and the rate-determining step of the reaction have not been clearly concluded. Here, the reaction mechanism on the Cu/ZnO/Al2O3, the Pd/ZnO, and the ZnZrOx catalysts was studied by in situ infrared spectroscopy and HCOOH temperature-programmed surface reaction (HCOOH-TPSR) experiment. It is shown that the HCOO* mechanism is a feasible mechanism, and the more stable HCOO* on the catalysts is, the higher the selectivity of methanol accompanied with the less CO produced via the decomposition of HCOO*. H2–D2 isotope exchange reaction is inhibited in the presence of CO2, which indicates that H2 activation and H* migration are inhibited by CO2 adsorbed on the catalysts. As for CO2 hydrogenation to methanol, the reaction orders of H2 and CO2 are close to 0.5 and 0, respectively, indicating that activated H* on the catalysts is insufficient. Comparing CO2 hydrogenation to methanol reaction and H2–D2 isotope exchange reaction, their H2 reaction orders are both 0.5 and the two reaction rates show a linear relationship when the temperature changes. It is considered that the rate-determining step of CO2 hydrogenation to methanol is the migration of H* on the catalysts.
中文翻译:

深入了解 CO2 加氢制甲醇的选择性决定因素和速率决定步骤
CO 2加氢制甲醇备受关注。该反应的机理、影响选择性的因素和限速步骤还没有明确的结论。在此,通过原位红外光谱和HCOOH程序升温表面反应(HCOOH-TPSR)实验研究了Cu/ZnO/Al 2 O 3、Pd/ZnO和ZnZrO x催化剂的反应机理。结果表明HCOO*机理是一种可行的机理,HCOO*在催化剂上越稳定,甲醇的选择性越高,同时HCOO*分解产生的CO越少。H 2 -D 2同位素交换反应在 CO 存在下受到抑制如图2所示,表明催化剂上吸附的CO 2抑制了H 2活化和H*迁移。对于CO 2加氢制甲醇,H 2和CO 2的反应级数分别接近0.5和0,表明催化剂上的活化H*不足。将CO 2加氢与甲醇反应和H 2 -D 2同位素交换反应进行比较,它们的H 2反应级数均为0.5,两种反应速率在温度变化时呈线性关系。认为CO 2的限速步骤氢化成甲醇是 H* 在催化剂上的迁移。
更新日期:2022-06-16
中文翻译:

深入了解 CO2 加氢制甲醇的选择性决定因素和速率决定步骤
CO 2加氢制甲醇备受关注。该反应的机理、影响选择性的因素和限速步骤还没有明确的结论。在此,通过原位红外光谱和HCOOH程序升温表面反应(HCOOH-TPSR)实验研究了Cu/ZnO/Al 2 O 3、Pd/ZnO和ZnZrO x催化剂的反应机理。结果表明HCOO*机理是一种可行的机理,HCOO*在催化剂上越稳定,甲醇的选择性越高,同时HCOO*分解产生的CO越少。H 2 -D 2同位素交换反应在 CO 存在下受到抑制如图2所示,表明催化剂上吸附的CO 2抑制了H 2活化和H*迁移。对于CO 2加氢制甲醇,H 2和CO 2的反应级数分别接近0.5和0,表明催化剂上的活化H*不足。将CO 2加氢与甲醇反应和H 2 -D 2同位素交换反应进行比较,它们的H 2反应级数均为0.5,两种反应速率在温度变化时呈线性关系。认为CO 2的限速步骤氢化成甲醇是 H* 在催化剂上的迁移。











































 京公网安备 11010802027423号
京公网安备 11010802027423号