European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2022-06-16 , DOI: 10.1016/j.ejmech.2022.114529 Bing Jiang 1 , Feng Han 1 , Ming-Huan Lü 1 , Zhen-Ping Wang 1 , Wei Liu 1 , Yun-Xiao Zhang 1 , Ji Xu 2 , Rui-Jun Li 1
|
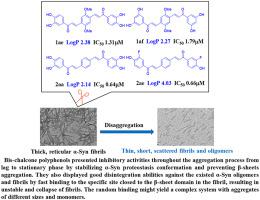
α-Syn fibrils, which are neurotoxic, play a key role in the development of PD. Maintaining α-Syn proteostasis by suitable molecule ligands is an effective approach to prevent aggregation. Disintegrating the existed oligomers and fibrils into individual α-Syn by small molecular compounds is a more efficient way to treat PD. This work designed and synthesized two series of bis-chalcone polyphenol compounds, which possess a sheet-like conjugated skeleton with stronger H-bonding, π-stacking, and hydrophobic interaction with α-Syn protein residues. Some compounds have shown high α-Syn aggregation inhibitory activities in vitro with IC50 down to 0.64 μM. The inhibition goes throughout the aggregation process from the lag to the stationary phase by stabilizing α-Syn proteostasis conformation and preventing β-sheets aggregation, especially in the lag phase. In addition, the inhibitors present good disintegration abilities against the existed α-Syn oligomers and fibrils. The preliminary mechanism studies suggest that the inhibitors could quickly and randomly bind to the specific site closed to the β-sheet domain in the fibril, resulting in unstable and collapse of the protein fibril and yielding a complex system with aggregates of different sizes and monomers. The inhibitors, which could penetrate the blood-brain barrier, are expected to develop into the drug candidates for PD targeting α-Syn aggregation.
中文翻译:

对 PD 具有潜在预防和治疗作用的双查耳酮多酚:针对 α-突触核蛋白低聚物和原纤维的设计、合成和体外解聚活性
具有神经毒性的 α-Syn 原纤维在 PD 的发展中起关键作用。通过合适的分子配体维持 α-Syn 蛋白稳态是防止聚集的有效方法。通过小分子化合物将现有的低聚物和原纤维分解成单独的α-Syn是一种更有效的治疗PD的方法。本工作设计并合成了两个系列的双查尔酮多酚化合物,它们具有片状共轭骨架,具有更强的氢键、π-堆积和与α-Syn蛋白残基的疏水相互作用。一些化合物在体外显示出较高的 α-Syn 聚集抑制活性,IC 50低至 0.64 μM。通过稳定 α-Syn 蛋白稳态构象和防止 β-折叠聚集,特别是在滞后期,抑制作用贯穿从滞后期到静止期的整个聚集过程。此外,抑制剂对现有的α-Syn低聚物和原纤维具有良好的崩解能力。初步机制研究表明,抑制剂可以快速、随机地结合到原纤维中靠近β-折叠结构域的特定位点,导致蛋白质原纤维不稳定和塌陷,并产生具有不同大小和单体聚集体的复杂系统。这些可以穿透血脑屏障的抑制剂有望发展成为靶向 α-Syn 聚集的 PD 候选药物。









































 京公网安备 11010802027423号
京公网安备 11010802027423号