当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
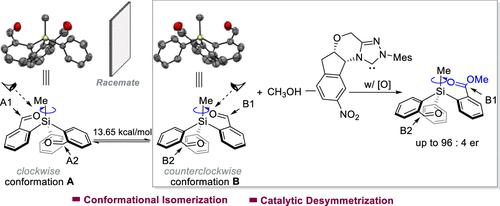
Construction of Tetrasubstituted Silicon-Stereogenic Silanes via Conformational Isomerization and N-Heterocyclic Carbene-Catalyzed Desymmetrization
ACS Catalysis ( IF 12.9 ) Pub Date : 2022-06-16 , DOI: 10.1021/acscatal.2c01082 Mali Zhou 1 , Jianjian Liu 1 , Rui Deng 1 , Qingyun Wang 1 , Shuquan Wu 1 , Pengcheng Zheng 1 , Yonggui Robin Chi 1, 2
ACS Catalysis ( IF 12.9 ) Pub Date : 2022-06-16 , DOI: 10.1021/acscatal.2c01082 Mali Zhou 1 , Jianjian Liu 1 , Rui Deng 1 , Qingyun Wang 1 , Shuquan Wu 1 , Pengcheng Zheng 1 , Yonggui Robin Chi 1, 2
Affiliation
|
Disclosed here is a catalytic strategy for asymmetric access to chiral tetrasubstituted silicon-stereogenic silanes. Our reaction starts with a (covalently) symmetric silane bearing two aldehyde moieties as the substrate. Single-crystal structural analysis shows that the substrate exists as a racemate of two conformational enantiomers because of the presence of a Si/O weak interaction. Mechanistic studies assisted by DFT calculation indicate that the two conformational enantiomers can readily isomerize to each other, and one of the conformational enantiomers of the substrate is favorably activated by a N-heterocyclic carbene catalyst via an overall desymmetrization process to eventually afford optically enriched tetrasubstituted silicon-stereogenic silanes as the products. Our chiral silanes’ products can be readily transformed to a diverse set of silicon stereogenic functional molecules.
中文翻译:

通过构象异构化和N-杂环卡宾催化的去对称化构建四取代硅-立体硅烷
这里公开了一种不对称获得手性四取代硅立体硅烷的催化策略。我们的反应从带有两个醛基的(共价)对称硅烷作为底物开始。单晶结构分析表明,由于存在Si/O弱相互作用,底物以两种构象对映异构体的外消旋体形式存在。由 DFT 计算辅助的机理研究表明,两种构象对映异构体可以很容易地相互异构化,并且底物的一种构象对映异构体被 N-杂环卡宾催化剂通过整体去对称化过程有利地活化,最终得到光学富集的四取代硅-作为产物的立体硅烷。
更新日期:2022-06-16
中文翻译:

通过构象异构化和N-杂环卡宾催化的去对称化构建四取代硅-立体硅烷
这里公开了一种不对称获得手性四取代硅立体硅烷的催化策略。我们的反应从带有两个醛基的(共价)对称硅烷作为底物开始。单晶结构分析表明,由于存在Si/O弱相互作用,底物以两种构象对映异构体的外消旋体形式存在。由 DFT 计算辅助的机理研究表明,两种构象对映异构体可以很容易地相互异构化,并且底物的一种构象对映异构体被 N-杂环卡宾催化剂通过整体去对称化过程有利地活化,最终得到光学富集的四取代硅-作为产物的立体硅烷。



























 京公网安备 11010802027423号
京公网安备 11010802027423号