当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible Light-Mediated, Highly Diastereoselective Epimerization of Lactams from the Most Accessible to the More Stable Stereoisomer
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-06-16 , DOI: 10.1021/acscatal.2c02232 Amaan M Kazerouni 1 , Daniel S Brandes 1 , Cassondra C Davies 2 , Laura F Cotter 1 , James M Mayer 1 , Shuming Chen 2 , Jonathan A Ellman 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-06-16 , DOI: 10.1021/acscatal.2c02232 Amaan M Kazerouni 1 , Daniel S Brandes 1 , Cassondra C Davies 2 , Laura F Cotter 1 , James M Mayer 1 , Shuming Chen 2 , Jonathan A Ellman 1
Affiliation
|
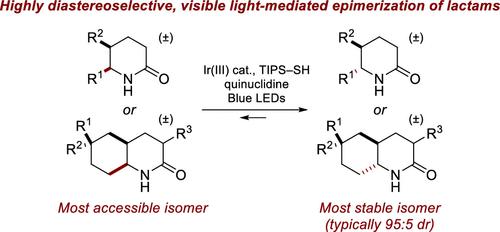
Most known methods to access δ-lactams with stereogenic centers at the α- and β-positions are highly selective for the contra-thermodynamic syn diastereomer, typically via hydrogenation of the corresponding pyridinones or quinolinones. We describe here the development of a photoredox-mediated hydrogen atom transfer approach for the epimerization of δ-lactams to access the more stable anti diastereomers from the contra-thermodynamic syn isomers. The reaction displays broad functional group compatibility, including acid, ester, 1°, 2°, and 3° amide, carbamate, and pyridyl groups, and was effective for a range of differently substituted monocyclic and bicyclic lactams. Experimentally observed diastereoselectivities are consistent with the calculated relative stabilities of lactam diastereomers. Convergence to the same diastereomer ratio from the syn- and anti-diastereomers establishes that reversible epimerization provides an equilibrium mixture of diastereomers. Additionally, deuterium labeling and luminescence quenching studies shed further light on the mechanism of the reaction.
中文翻译:

可见光介导的内酰胺从最容易接近的立体异构体到更稳定的立体异构体的高度非对映选择性差向异构化
大多数已知的获取在α-和β-位具有立体中心的δ-内酰胺的方法对于反热力学顺式非对映异构体具有高度选择性,通常通过相应吡啶酮或喹啉酮的氢化来实现。我们在这里描述了光氧化还原介导的氢原子转移方法的发展,用于δ-内酰胺的差向异构化,以从反热力学顺式中获得更稳定的反非对映异构体异构体。该反应表现出广泛的官能团兼容性,包括酸、酯、1°、2°和3°酰胺、氨基甲酸酯和吡啶基,并且对一系列不同取代的单环和双环内酰胺有效。实验观察到的非对映选择性与计算的内酰胺非对映体的相对稳定性一致。顺式非对映体和反式非对映体收敛到相同的非对映体比率表明可逆差向异构化提供了非对映体的平衡混合物。此外,氘标记和发光猝灭研究进一步阐明了反应机制。
更新日期:2022-06-16
中文翻译:

可见光介导的内酰胺从最容易接近的立体异构体到更稳定的立体异构体的高度非对映选择性差向异构化
大多数已知的获取在α-和β-位具有立体中心的δ-内酰胺的方法对于反热力学顺式非对映异构体具有高度选择性,通常通过相应吡啶酮或喹啉酮的氢化来实现。我们在这里描述了光氧化还原介导的氢原子转移方法的发展,用于δ-内酰胺的差向异构化,以从反热力学顺式中获得更稳定的反非对映异构体异构体。该反应表现出广泛的官能团兼容性,包括酸、酯、1°、2°和3°酰胺、氨基甲酸酯和吡啶基,并且对一系列不同取代的单环和双环内酰胺有效。实验观察到的非对映选择性与计算的内酰胺非对映体的相对稳定性一致。顺式非对映体和反式非对映体收敛到相同的非对映体比率表明可逆差向异构化提供了非对映体的平衡混合物。此外,氘标记和发光猝灭研究进一步阐明了反应机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号