当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chichibabin-Type Phosphonylation of 2-(Hetero)aryl Pyridines: Selective Synthesis of 4-Phosphinoyl Pyridines via an Activated N-Benzylpyridinium Salt
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-06-15 , DOI: 10.1002/adsc.202200289 Xiao Yang 1 , Rui Sun 1 , Chunchun Zhang 2 , Yan Zhang 1 , Zhishan Su 3 , Yicen Ge 4 , Hua Chen 2 , haiyan Fu 2 , Ruixiang Li 5
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-06-15 , DOI: 10.1002/adsc.202200289 Xiao Yang 1 , Rui Sun 1 , Chunchun Zhang 2 , Yan Zhang 1 , Zhishan Su 3 , Yicen Ge 4 , Hua Chen 2 , haiyan Fu 2 , Ruixiang Li 5
Affiliation
|
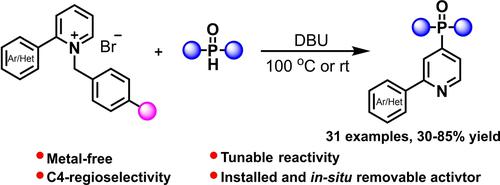
A direct C−H phosphonylation of pyridine by N-benzylating activation is reported, which afforded 4-pyridylphosphine oxides with high regioselectivity, without the employment of metal catalyst or expensive activator. By increasing the electrophilicity of pyridinium with electron-withdrawing substituents on the N-benzyl group, the phosphonylation process could be realized at room temperature. Control experiments and NMR investigation confirmed the interaction between DBU and diphenylphosphine oxide which generated the true phosphorus nucleophile. Moreover, DFT calculations offered enough insight into an intermolecular cooperative dehydrogenation-debenzylation mechanism, which helped to elucidate the origin of C4-regioselectivity in this metal-free Chichibabin-type phosphonylation.
中文翻译:

2-(杂)芳基吡啶的 Chichibabin 型膦酰化:通过活化的 N-苄基吡啶鎓盐选择性合成 4-膦酰基吡啶
报道了通过N-苄基化活化对吡啶进行直接 C-H 膦酰化,无需使用金属催化剂或昂贵的活化剂,即可得到具有高区域选择性的 4-吡啶基氧化膦。通过在N-苄基上增加吸电子取代基的吡啶鎓的亲电性,可以在室温下实现膦酰化过程。对照实验和 NMR 研究证实了 DBU 和二苯基氧化膦之间的相互作用,从而产生了真正的磷亲核试剂。此外,DFT 计算为分子间协同脱氢-脱苄基化机制提供了足够的洞察力,这有助于阐明这种无金属 Chichibabin 型膦酰化中 C4 区域选择性的起源。
更新日期:2022-06-15
中文翻译:

2-(杂)芳基吡啶的 Chichibabin 型膦酰化:通过活化的 N-苄基吡啶鎓盐选择性合成 4-膦酰基吡啶
报道了通过N-苄基化活化对吡啶进行直接 C-H 膦酰化,无需使用金属催化剂或昂贵的活化剂,即可得到具有高区域选择性的 4-吡啶基氧化膦。通过在N-苄基上增加吸电子取代基的吡啶鎓的亲电性,可以在室温下实现膦酰化过程。对照实验和 NMR 研究证实了 DBU 和二苯基氧化膦之间的相互作用,从而产生了真正的磷亲核试剂。此外,DFT 计算为分子间协同脱氢-脱苄基化机制提供了足够的洞察力,这有助于阐明这种无金属 Chichibabin 型膦酰化中 C4 区域选择性的起源。











































 京公网安备 11010802027423号
京公网安备 11010802027423号