当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modeling of Catalyst Deactivation in Humic Acid Degradation
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2022-06-16 , DOI: 10.1021/acs.iecr.2c00837 Leandro G. Aguiar 1 , Adriano F. Siqueira 1
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2022-06-16 , DOI: 10.1021/acs.iecr.2c00837 Leandro G. Aguiar 1 , Adriano F. Siqueira 1
Affiliation
|
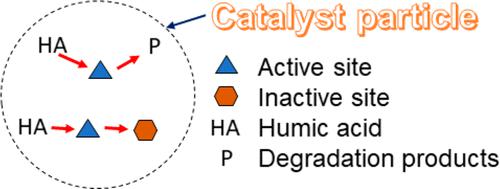
A kinetic model is proposed to describe humic acid (HA) degradation by TiO2- and ZnO-based catalysts taking into account the effects of adsorption and catalyst deactivation. The model was written in Scilab and validated with data from 14 references and 60 experiments, providing an R2 value of 0.925. The induction time observed in some experiments was also considered in the model, improving predictions. The rate constant for HA degradation showed feasible values compared with the first-order kinetic constants found in the literature. This parameter indicated that the efficiency of TiO2-based catalysts was slightly higher than that of ZnO-based catalysts. For experiments using a minimum of 100 mg L–1 catalyst, the average specific rates of 1.18 × 10–4 and 3.27 × 10–3 L mg–1 min–1 were found for HA degradation and catalyst deactivation, respectively. Furthermore, the analytical solution of the model provided an equation to determine the maximum HA degradation as a function of initial experimental conditions.
中文翻译:

腐殖酸降解中催化剂失活的建模
考虑到吸附和催化剂失活的影响,提出了一个动力学模型来描述 TiO 2 - 和 ZnO 基催化剂对腐殖酸 (HA) 的降解。该模型是在 Scilab 中编写的,并使用来自 14 个参考文献和 60 个实验的数据进行了验证,提供了0.925 的R 2值。模型中还考虑了在一些实验中观察到的诱导时间,从而改进了预测。与文献中发现的一级动力学常数相比,HA 降解的速率常数显示出可行的值。该参数表明基于 TiO 2的催化剂的效率略高于基于 ZnO 的催化剂。对于使用至少 100 mg L –1的实验催化剂, HA 降解和催化剂失活的平均比速率分别为 1.18 × 10 –4和 3.27 × 10 –3 L mg –1 min –1 。此外,模型的解析解提供了一个方程来确定作为初始实验条件函数的最大 HA 降解。
更新日期:2022-06-16
中文翻译:

腐殖酸降解中催化剂失活的建模
考虑到吸附和催化剂失活的影响,提出了一个动力学模型来描述 TiO 2 - 和 ZnO 基催化剂对腐殖酸 (HA) 的降解。该模型是在 Scilab 中编写的,并使用来自 14 个参考文献和 60 个实验的数据进行了验证,提供了0.925 的R 2值。模型中还考虑了在一些实验中观察到的诱导时间,从而改进了预测。与文献中发现的一级动力学常数相比,HA 降解的速率常数显示出可行的值。该参数表明基于 TiO 2的催化剂的效率略高于基于 ZnO 的催化剂。对于使用至少 100 mg L –1的实验催化剂, HA 降解和催化剂失活的平均比速率分别为 1.18 × 10 –4和 3.27 × 10 –3 L mg –1 min –1 。此外,模型的解析解提供了一个方程来确定作为初始实验条件函数的最大 HA 降解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号