当前位置:
X-MOL 学术
›
Pharmacol. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kidney Angiotensin in Cardiovascular Disease: Formation and Drug Targeting
Pharmacological Reviews ( IF 19.3 ) Pub Date : 2022-07-01 , DOI: 10.1124/pharmrev.120.000236 Hui Lin 1 , Frank Geurts 1 , Luise Hassler 1 , Daniel Batlle 1 , Katrina M Mirabito Colafella 1 , Kate M Denton 1 , Jia L Zhuo 1 , Xiao C Li 1 , Nirupama Ramkumar 1 , Masahiro Koizumi 1 , Taiji Matsusaka 1 , Akira Nishiyama 1 , Martin J Hoogduijn 1 , Ewout J Hoorn 1 , A H Jan Danser 1
Pharmacological Reviews ( IF 19.3 ) Pub Date : 2022-07-01 , DOI: 10.1124/pharmrev.120.000236 Hui Lin 1 , Frank Geurts 1 , Luise Hassler 1 , Daniel Batlle 1 , Katrina M Mirabito Colafella 1 , Kate M Denton 1 , Jia L Zhuo 1 , Xiao C Li 1 , Nirupama Ramkumar 1 , Masahiro Koizumi 1 , Taiji Matsusaka 1 , Akira Nishiyama 1 , Martin J Hoogduijn 1 , Ewout J Hoorn 1 , A H Jan Danser 1
Affiliation
|
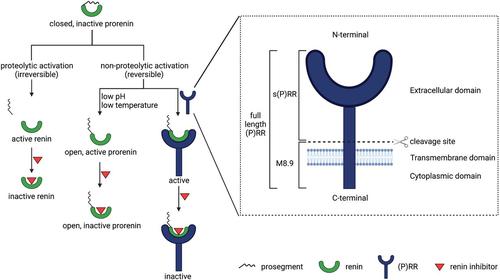
The concept of local formation of angiotensin II in the kidney has changed over the last 10–15 years. Local synthesis of angiotensinogen in the proximal tubule has been proposed, combined with prorenin synthesis in the collecting duct. Binding of prorenin via the so-called (pro)renin receptor has been introduced, as well as megalin-mediated uptake of filtered plasma-derived renin-angiotensin system (RAS) components. Moreover, angiotensin metabolites other than angiotensin II [notably angiotensin-(1-7)] exist, and angiotensins exert their effects via three different receptors, of which angiotensin II type 2 and Mas receptors are considered renoprotective, possibly in a sex-specific manner, whereas angiotensin II type 1 (AT1) receptors are believed to be deleterious. Additionally, internalized angiotensin II may stimulate intracellular receptors. Angiotensin-converting enzyme 2 (ACE2) not only generates angiotensin-(1-7) but also acts as coronavirus receptor. Multiple, if not all, cardiovascular diseases involve the kidney RAS, with renal AT1 receptors often being claimed to exert a crucial role. Urinary RAS component levels, depending on filtration, reabsorption, and local release, are believed to reflect renal RAS activity. Finally, both existing drugs (RAS inhibitors, cyclooxygenase inhibitors) and novel drugs (angiotensin receptor/neprilysin inhibitors, sodium-glucose cotransporter-2 inhibitors, soluble ACE2) affect renal angiotensin formation, thereby displaying cardiovascular efficacy. Particular in the case of the latter three, an important question is to what degree they induce renoprotection (e.g., in a renal RAS-dependent manner). This review provides a unifying view, explaining not only how kidney angiotensin formation occurs and how it is affected by drugs but also why drugs are renoprotective when altering the renal RAS.
中文翻译:

心血管疾病中的肾血管紧张素:形成和药物靶向
在过去 10-15 年中,肾脏局部形成血管紧张素 II 的概念发生了变化。已提出在近曲小管中局部合成血管紧张素原,并结合集合管中的肾素原合成。已经引入了肾素原通过所谓的肾素原受体的结合,以及巨蛋白介导的过滤血浆来源的肾素-血管紧张素系统(RAS)成分的摄取。此外,还存在除血管紧张素 II [特别是血管紧张素-(1-7)] 以外的血管紧张素代谢物,并且血管紧张素通过三种不同的受体发挥作用,其中血管紧张素 II 2 型和 Mas 受体被认为具有肾脏保护作用,可能以性别特异性的方式,而血管紧张素 II 1 型 (AT 1 ) 受体被认为是有害的。此外,内化的血管紧张素 II 可能会刺激细胞内受体。血管紧张素转换酶 2 (ACE2) 不仅产生血管紧张素-(1-7),还充当冠状病毒受体。多种(如果不是全部)心血管疾病都涉及肾脏 RAS,而肾脏 AT 1受体通常被认为发挥着至关重要的作用。尿液 RAS 成分水平取决于过滤、重吸收和局部释放,被认为反映了肾脏 RAS 活性。最后,现有药物(RAS抑制剂、环氧合酶抑制剂)和新药(血管紧张素受体/脑啡肽酶抑制剂、钠-葡萄糖协同转运蛋白2抑制剂、可溶性ACE2)都会影响肾血管紧张素的形成,从而显示出心血管疗效。特别是后三种情况,一个重要的问题是它们在多大程度上诱导肾脏保护(例如,以肾 RAS 依赖性方式)。 这篇综述提供了一个统一的观点,不仅解释了肾脏血管紧张素的形成如何发生以及药物如何影响它,还解释了为什么药物在改变肾脏 RAS 时具有肾脏保护作用。
更新日期:2022-06-16
中文翻译:

心血管疾病中的肾血管紧张素:形成和药物靶向
在过去 10-15 年中,肾脏局部形成血管紧张素 II 的概念发生了变化。已提出在近曲小管中局部合成血管紧张素原,并结合集合管中的肾素原合成。已经引入了肾素原通过所谓的肾素原受体的结合,以及巨蛋白介导的过滤血浆来源的肾素-血管紧张素系统(RAS)成分的摄取。此外,还存在除血管紧张素 II [特别是血管紧张素-(1-7)] 以外的血管紧张素代谢物,并且血管紧张素通过三种不同的受体发挥作用,其中血管紧张素 II 2 型和 Mas 受体被认为具有肾脏保护作用,可能以性别特异性的方式,而血管紧张素 II 1 型 (AT 1 ) 受体被认为是有害的。此外,内化的血管紧张素 II 可能会刺激细胞内受体。血管紧张素转换酶 2 (ACE2) 不仅产生血管紧张素-(1-7),还充当冠状病毒受体。多种(如果不是全部)心血管疾病都涉及肾脏 RAS,而肾脏 AT 1受体通常被认为发挥着至关重要的作用。尿液 RAS 成分水平取决于过滤、重吸收和局部释放,被认为反映了肾脏 RAS 活性。最后,现有药物(RAS抑制剂、环氧合酶抑制剂)和新药(血管紧张素受体/脑啡肽酶抑制剂、钠-葡萄糖协同转运蛋白2抑制剂、可溶性ACE2)都会影响肾血管紧张素的形成,从而显示出心血管疗效。特别是后三种情况,一个重要的问题是它们在多大程度上诱导肾脏保护(例如,以肾 RAS 依赖性方式)。 这篇综述提供了一个统一的观点,不仅解释了肾脏血管紧张素的形成如何发生以及药物如何影响它,还解释了为什么药物在改变肾脏 RAS 时具有肾脏保护作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号