当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carbonate-Ion-Mediated Photogenerated Hole Transfer to Boost Hydrogen Production
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2022-06-16 , DOI: 10.1021/acs.jpcc.2c02252 Peng Yu 1, 2 , Jun Meng 3 , Fengmei Wang 1 , Marshet Getaye Sendeku 1 , Binglan Wu 1 , Xinyu Sui 4 , Ning Gao 1 , Xueying Zhan 1 , Xiaoding Lou 2 , Zhenxing Wang 1 , Jun He 5
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2022-06-16 , DOI: 10.1021/acs.jpcc.2c02252 Peng Yu 1, 2 , Jun Meng 3 , Fengmei Wang 1 , Marshet Getaye Sendeku 1 , Binglan Wu 1 , Xinyu Sui 4 , Ning Gao 1 , Xueying Zhan 1 , Xiaoding Lou 2 , Zhenxing Wang 1 , Jun He 5
Affiliation
|
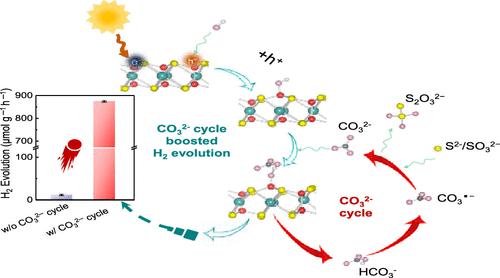
Sustainable and scalable H2 evolution through water photocatalysis is an attractive path for carbon-neutral energy supply; however, it is severely limited by sluggish charge separation and photocorrosion of semiconductor photocatalysts. Here, we demonstrate that earth-abundant carbonate ions, widely existing in daily-life water, serve as a hole mediator to redirect the photogenerated hole transfer pathway and then promote the hole-transfer kinetics. The accelerated hole transfer could efficiently reduce the recombination of electron–hole pairs for continuous H2 production with improved photostability of catalysts, including layered indium phosphorus sulfide (In4/3P2S6) and cadmium sulfide. A sustainable H2 evolution rate of 5.1 mmol g–1 h–1 within 60 h or more operation is achieved in the presence of CO32– anions. In situ electron spin resonance (ESR) spectroscopy studies and transient absorption (TA) measurements reveal that the CO32–/CO3●– redox couple could rapidly shuttle the photogenerated holes from OH● radicals anchored on the catalyst surface, effectively eliminating the recombination of electron–hole pairs and catalyst oxidation for boosted H2 generation. The carbonate-ion-mediated hole-transfer strategy provides a new paradigm for designing a cost-effective and advanced photosynthetic system in practical applications.
中文翻译:

碳酸盐离子介导的光生空穴转移促进制氢
通过水光催化产生可持续和可扩展的 H 2是碳中和能源供应的一条有吸引力的途径;然而,它受到缓慢的电荷分离和半导体光催化剂的光腐蚀的严重限制。在这里,我们证明了广泛存在于日常生活水中的地球丰富的碳酸根离子作为空穴介体来改变光生空穴传输路径,然后促进空穴传输动力学。加速的空穴转移可以有效地减少电子-空穴对的复合,从而提高催化剂的光稳定性,包括层状氧化铟磷硫化物(In 4/3 P 2 S 6 )和硫化镉。可持续的 H在CO 3 2-阴离子存在的情况下,在60 小时或更长时间的操作中实现了5.1 mmol g –1 h –1的2释放速率。原位电子自旋共振 (ESR) 光谱研究和瞬态吸收 (TA) 测量表明,CO 3 2– /CO 3 ●–氧化还原对可以快速穿梭与锚定在催化剂表面的 OH ●自由基的光生空穴,有效消除电子-空穴对的重组和催化剂氧化促进 H 2一代。碳酸盐离子介导的空穴转移策略为在实际应用中设计具有成本效益和先进的光合系统提供了新的范例。
更新日期:2022-06-16
中文翻译:

碳酸盐离子介导的光生空穴转移促进制氢
通过水光催化产生可持续和可扩展的 H 2是碳中和能源供应的一条有吸引力的途径;然而,它受到缓慢的电荷分离和半导体光催化剂的光腐蚀的严重限制。在这里,我们证明了广泛存在于日常生活水中的地球丰富的碳酸根离子作为空穴介体来改变光生空穴传输路径,然后促进空穴传输动力学。加速的空穴转移可以有效地减少电子-空穴对的复合,从而提高催化剂的光稳定性,包括层状氧化铟磷硫化物(In 4/3 P 2 S 6 )和硫化镉。可持续的 H在CO 3 2-阴离子存在的情况下,在60 小时或更长时间的操作中实现了5.1 mmol g –1 h –1的2释放速率。原位电子自旋共振 (ESR) 光谱研究和瞬态吸收 (TA) 测量表明,CO 3 2– /CO 3 ●–氧化还原对可以快速穿梭与锚定在催化剂表面的 OH ●自由基的光生空穴,有效消除电子-空穴对的重组和催化剂氧化促进 H 2一代。碳酸盐离子介导的空穴转移策略为在实际应用中设计具有成本效益和先进的光合系统提供了新的范例。











































 京公网安备 11010802027423号
京公网安备 11010802027423号