当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of Biocompatible Ene-Ligation Enabled by Prenyl-Based β-Caryophyllene with Triazoline/Selectfluor under Physiological Conditions
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-16 , DOI: 10.1021/acs.joc.2c00841 Sheng Wang 1 , Yuanyuan Li 1 , Hongling Zhou 1 , Li Wang 2 , Rui Wang 1, 3
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-16 , DOI: 10.1021/acs.joc.2c00841 Sheng Wang 1 , Yuanyuan Li 1 , Hongling Zhou 1 , Li Wang 2 , Rui Wang 1, 3
Affiliation
|
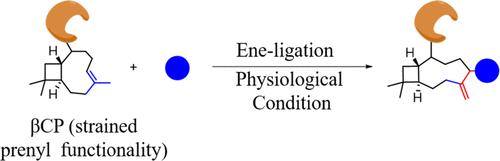
Here, we first report a rapid and highly selective biocompatible ligation that proceeds via a strain-promoted prenyl-involved [2, 3]-Ene rearrangement process. We demonstrate the usefulness of naturally occurring strain-promoted β-caryophyllene with triazoline (PTAD)/Selectfluor in the study of tagging molecule-of-interest. Experiments in peptide (Histone H3 (1–21) and Myhc (614–629)) and protein (BSA, βLG, and HSP40) models exemplified the utility of the Ene-ligation for in vivo imaging and tracking.
中文翻译:

生理条件下异戊烯基 β-石竹烯与三唑啉/Selectfluor 实现生物相容性烯连接的发展
在这里,我们首先报告了一种快速且高度选择性的生物相容性连接,该连接通过菌株促进的异戊二烯参与的 [2, 3]-烯重排过程进行。我们证明了天然存在的菌株促进的 β-石竹烯与三唑啉 (PTAD)/Selectfluor 在标记感兴趣分子的研究中的有用性。肽(组蛋白 H3 (1-21) 和 Myhc (614-629))和蛋白质(BSA、βLG 和 HSP40)模型的实验证明了 Ene-ligation 在体内成像和跟踪中的实用性。
更新日期:2022-06-16
中文翻译:

生理条件下异戊烯基 β-石竹烯与三唑啉/Selectfluor 实现生物相容性烯连接的发展
在这里,我们首先报告了一种快速且高度选择性的生物相容性连接,该连接通过菌株促进的异戊二烯参与的 [2, 3]-烯重排过程进行。我们证明了天然存在的菌株促进的 β-石竹烯与三唑啉 (PTAD)/Selectfluor 在标记感兴趣分子的研究中的有用性。肽(组蛋白 H3 (1-21) 和 Myhc (614-629))和蛋白质(BSA、βLG 和 HSP40)模型的实验证明了 Ene-ligation 在体内成像和跟踪中的实用性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号