当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regioselective Cycloaddition and Substitution Reaction of Tertiary Propargylic Alcohols and Heteroareneboronic Acids via Acid Catalysis
Organic Letters ( IF 4.9 ) Pub Date : 2022-06-15 , DOI: 10.1021/acs.orglett.2c01403 Jian-Fei Bai 1 , Jianbo Tang 1 , Xiaolong Gao 2 , Zhi-Jiang Jiang 1 , Bencan Tang 3 , Jia Chen 1 , Zhanghua Gao 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-06-15 , DOI: 10.1021/acs.orglett.2c01403 Jian-Fei Bai 1 , Jianbo Tang 1 , Xiaolong Gao 2 , Zhi-Jiang Jiang 1 , Bencan Tang 3 , Jia Chen 1 , Zhanghua Gao 1
Affiliation
|
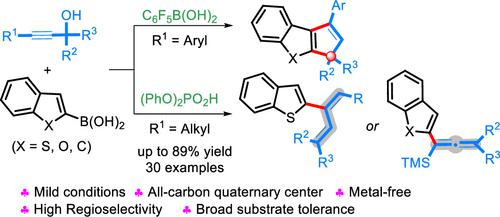
We report an acid-catalyzed formal cycloaddition and dehydrative substitution reaction of tertiary propargylic alcohols and heteroareneboronic acids. The properties of the substituents on the alkynyl moiety determines the regioselectivity of the reaction, which could selectively construct fused heterocycles, tetrasubstituted allenes, or 1,3-dienes. This reaction proceeds efficiently with a wide array of substrate scope in up to 89% yield. A significant advantage of this protocol is the transition-metal-free and mild conditions needed.
中文翻译:

酸催化炔丙醇与杂芳烃硼酸的区域选择性环加成和取代反应
我们报告了叔炔丙醇和杂芳烃硼酸的酸催化形式环加成和脱水取代反应。炔基部分取代基的性质决定了反应的区域选择性,可以选择性地构建稠合杂环、四取代丙二烯或1,3-二烯。该反应在广泛的底物范围内高效进行,产率高达 89%。该协议的一个显着优势是所需的无过渡金属和温和条件。
更新日期:2022-06-15
中文翻译:

酸催化炔丙醇与杂芳烃硼酸的区域选择性环加成和取代反应
我们报告了叔炔丙醇和杂芳烃硼酸的酸催化形式环加成和脱水取代反应。炔基部分取代基的性质决定了反应的区域选择性,可以选择性地构建稠合杂环、四取代丙二烯或1,3-二烯。该反应在广泛的底物范围内高效进行,产率高达 89%。该协议的一个显着优势是所需的无过渡金属和温和条件。











































 京公网安备 11010802027423号
京公网安备 11010802027423号