当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Physico-geometrical kinetics of the thermal dehydration of sodium carbonate monohydrate as a compacted composite of inorganic hydrate comprising crystalline particles and matrix
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2022-06-16 , DOI: 10.1039/d2cp01948e Yuto Zushi 1 , Shun Iwasaki 1 , Nobuyoshi Koga 1
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2022-06-16 , DOI: 10.1039/d2cp01948e Yuto Zushi 1 , Shun Iwasaki 1 , Nobuyoshi Koga 1
Affiliation
|
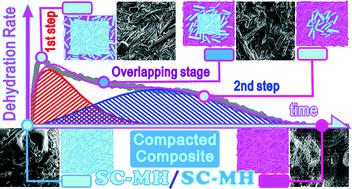
The kinetics of the thermal dehydration of compacted composite grains of Na2CO3·H2O (SC-MH) comprising columnar SC-MH crystalline particles and an SC-MH matrix were investigated as a model system for composites of the same compound with a porphyritic texture. The presence of an induction period was confirmed as a novel finding for the thermal dehydration of SC-MH. The subsequent mass loss process was characterized as a partially overlapping two-step process attributed to the consecutive reactions of SC-MH matrix and columnar SC-MH crystalline particles. The overlapping nature of two reaction steps was revealed by determining the contributions and kinetic parameters of the individual reaction steps via a kinetic deconvolution analysis. Furthermore, the initial mass loss process caused by the thermal dehydration of the SC-MH matrix was characterized as a physico-geometrical consecutive process comprising a surface reaction and a subsequent three-dimensional (3D)-phase boundary-controlled reaction. The subsequent thermal dehydration of the columnar SC-MH crystalline particles compacted in the grains was characterized as being geometrically constrained by 3D-interface shrinkage, forming two reaction interfaces during the overlapping stage of the two reaction steps. It was expected from the kinetic results that the linear advancement rate of the second reaction interface was influenced by the water vapor produced at the reaction interface of the first reaction step. This caused the linear advancement rate of the second reaction interface to accelerate as the reaction proceeded due to contraction of the first reaction interface and completion of the first reaction step.
中文翻译:

碳酸钠一水合物热脱水的物理几何动力学作为无机水合物的致密复合物,包括晶体颗粒和基质
由柱状 SC-MH 晶体颗粒和 SC-MH 基体组成的 Na 2 CO 3 ·H 2 O (SC-MH)压实复合颗粒的热脱水动力学作为相同化合物复合材料的模型系统进行了研究。斑状纹理。诱导期的存在被证实是 SC-MH 热脱水的新发现。随后的质量损失过程被表征为部分重叠的两步过程,归因于 SC-MH 基质和柱状 SC-MH 晶体颗粒的连续反应。通过确定各个反应步骤的贡献和动力学参数,揭示了两个反应步骤的重叠性质动力学反卷积分析。此外,由 SC-MH 基质的热脱水引起的初始质量损失过程被表征为物理几何连续过程,包括表面反应和随后的三维 (3D) 相边界控制反应。随后压缩在晶粒中的柱状 SC-MH 晶体颗粒的热脱水被表征为受到 3D 界面收缩的几何约束,在两个反应步骤的重叠阶段形成两个反应界面。从动力学结果可以看出,第二反应界面的线性推进速率受第一反应步骤的反应界面处产生的水蒸气的影响。
更新日期:2022-06-16
中文翻译:

碳酸钠一水合物热脱水的物理几何动力学作为无机水合物的致密复合物,包括晶体颗粒和基质
由柱状 SC-MH 晶体颗粒和 SC-MH 基体组成的 Na 2 CO 3 ·H 2 O (SC-MH)压实复合颗粒的热脱水动力学作为相同化合物复合材料的模型系统进行了研究。斑状纹理。诱导期的存在被证实是 SC-MH 热脱水的新发现。随后的质量损失过程被表征为部分重叠的两步过程,归因于 SC-MH 基质和柱状 SC-MH 晶体颗粒的连续反应。通过确定各个反应步骤的贡献和动力学参数,揭示了两个反应步骤的重叠性质动力学反卷积分析。此外,由 SC-MH 基质的热脱水引起的初始质量损失过程被表征为物理几何连续过程,包括表面反应和随后的三维 (3D) 相边界控制反应。随后压缩在晶粒中的柱状 SC-MH 晶体颗粒的热脱水被表征为受到 3D 界面收缩的几何约束,在两个反应步骤的重叠阶段形成两个反应界面。从动力学结果可以看出,第二反应界面的线性推进速率受第一反应步骤的反应界面处产生的水蒸气的影响。









































 京公网安备 11010802027423号
京公网安备 11010802027423号