Journal of Hepatology ( IF 25.7 ) Pub Date : 2022-06-15 , DOI: 10.1016/j.jhep.2022.05.040 Nadia Bresciani 1 , Hadrien Demagny 1 , Vera Lemos 1 , Francesca Pontanari 1 , Xiaoxu Li 2 , Yu Sun 1 , Hao Li 3 , Alessia Perino 1 , Johan Auwerx 2 , Kristina Schoonjans 1
|
Background & Aims
Transporters of the SLC25 mitochondrial carrier superfamily bridge cytoplasmic and mitochondrial metabolism by channeling metabolites across mitochondrial membranes and are pivotal for metabolic homeostasis. Despite their physiological relevance as gatekeepers of cellular metabolism, most of the SLC25 family members remain uncharacterized. We undertook a comprehensive tissue distribution analysis of all Slc25 family members across metabolic organs and identified SLC25A47 as a liver-specific mitochondrial carrier.
Methods
We used a murine loss-of-function model to unravel the role of this transporter in mitochondrial and hepatic homeostasis. We performed extensive metabolic phenotyping and molecular characterization of newly generated Slc25a47hep-/- and Slc25a47-Fgf21hep-/- mice.
Results
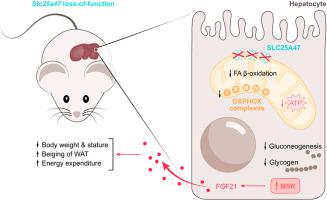
Slc25a47hep-/- mice displayed a wide variety of metabolic abnormalities, as a result of sustained energy deficiency in the liver originating from impaired mitochondrial respiration. This mitochondrial phenotype was associated with an activation of the mitochondrial stress response (MSR) in the liver, and the development of fibrosis, which was exacerbated upon feeding a high-fat high-sucrose diet. The MSR induced the secretion of several mitokines, amongst which FGF21 played a preponderant role on systemic physiology. To dissect the FGF21-dependent and -independent physiological changes induced in Slc25a47hep-/- mice, we generated a double Slc25a47-Fgf21hep-/- mouse model and demonstrated that several aspects of the hypermetabolic state were driven by hepatic secretion of FGF21. On the other hand, the metabolic fuel inflexibility observed in Slc25a47hep-/- mice could not be rescued with the genetic removal of Fgf21.
Conclusion
Collectively, our data place the Slc25a47 locus at the center of mitochondrial homeostasis, which upon dysfunction triggers robust liver-specific and systemic adaptive stress responses. The prominent role of the Slc25a47 locus in hepatic fibrosis identifies this carrier, or its transported metabolite, as a potential target for therapeutic intervention.
Lay summary
Herein, we report the importance of a locus containing a liver-specific gene coding for a mitochondrial transport protein called SLC25A47. Mitochondria are the powerhouses of cells. They are crucial for metabolism and energy generation. We show that mice with genetic disruption of the Slc25a47 locus cannot maintain mitochondrial homeostasis (balance), leading to wide-ranging problems in the liver that have far-reaching physiological consequences.
中文翻译:

Slc25a47 基因座是与肝纤维化有关的肝线粒体功能的新决定因素
背景与目标
SLC25 线粒体载体超家族的转运蛋白通过引导代谢物穿过线粒体膜来桥接细胞质和线粒体代谢,并且对于代谢稳态至关重要。尽管它们作为细胞代谢的看门人具有生理相关性,但大多数 SLC25 家族成员仍未被表征。我们对代谢器官中所有 SLC25 家族成员进行了全面的组织分布分析,并将 SLC25A47 鉴定为肝脏特异性线粒体载体。
方法
我们使用小鼠功能丧失模型来揭示这种转运蛋白在线粒体和肝脏稳态中的作用。我们对新生成的Slc25a47 hep-/-和Slc25a47-Fgf21 hep-/-小鼠进行了广泛的代谢表型分析和分子表征。
结果
Slc25a47 hep- /-小鼠表现出多种代谢异常,这是由于线粒体呼吸受损导致肝脏持续能量缺乏的结果。这种线粒体表型与肝脏中线粒体应激反应 (MSR) 的激活以及纤维化的发展有关,在喂食高脂肪高蔗糖饮食后会加剧这种情况。MSR诱导了几种有丝分裂因子的分泌,其中FGF21在全身生理学中起主要作用。为了剖析在Slc25a47 hep-/-小鼠中诱导的 FGF21 依赖性和非依赖性生理变化,我们生成了双Slc25a47 - Fgf21 hep-/-小鼠模型并证明高代谢状态的几个方面是由 FGF21 的肝脏分泌驱动的。另一方面,在Slc25a47 hep-/-小鼠中观察到的代谢燃料不灵活不能通过Fgf21的基因去除来挽救。
结论
总的来说,我们的数据将Slc25a47基因座置于线粒体稳态的中心,一旦功能障碍触发强大的肝脏特异性和全身适应性应激反应。Slc25a47基因座在肝纤维化中的突出作用将这种载体或其转运代谢物确定为治疗干预的潜在靶标。
总结
在这里,我们报告了一个包含肝脏特异性基因的基因座的重要性,该基因编码称为 SLC25A47 的线粒体转运蛋白。线粒体是细胞的动力源。它们对新陈代谢和能量产生至关重要。我们表明,Slc25a47基因座遗传破坏的小鼠无法维持线粒体稳态(平衡),从而导致肝脏出现广泛问题,并产生深远的生理后果。



























 京公网安备 11010802027423号
京公网安备 11010802027423号