当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of a PPAP, Nemorosonol, Using a Tandem Michael Addition–Intramolecular Aldol Reaction
Organic Letters ( IF 4.9 ) Pub Date : 2022-06-15 , DOI: 10.1021/acs.orglett.2c01745 Keisuke Mitsugi 1 , Toru Takabayashi 1 , Takayuki Ohyoshi 1 , Hideo Kigoshi 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-06-15 , DOI: 10.1021/acs.orglett.2c01745 Keisuke Mitsugi 1 , Toru Takabayashi 1 , Takayuki Ohyoshi 1 , Hideo Kigoshi 1
Affiliation
|
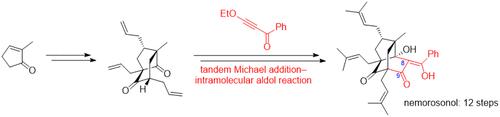
A strategy for constructing a tricyclo[4.3.1.03,7]decane skeleton, which is common to many polycyclic polyprenylated acylphloroglucinols, has been established. The key step was a tandem Michael addition–intramolecular aldol reaction with 3-ethoxy-1-phenyl-2-proyn-1-one, which affords a tricyclo[4.3.1.03,7]decane skeleton having a benzoyl group at the C8 position and an appropriate oxygen functional group at the C9 position. This synthetic strategy led to the total synthesis of nemorosonol, which was accomplished in 12 steps from 2-methyl-2-cyclopenten-1-one.
中文翻译:

PPAP、Nemorosonol 的全合成,使用串联迈克尔加成 - 分子内羟醛反应
已经建立了构建三环[4.3.1.0 3,7 ]癸烷骨架的策略,这是许多多环聚异戊二烯化酰基间苯三酚共有的骨架。关键步骤是串联迈克尔加成 - 与 3-ethoxy-1-phenyl-2-proyn-1-one 的分子内醛醇反应,得到在 C8 上具有苯甲酰基的三环[4.3.1.0 3,7 ]癸烷骨架位置和适当的氧官能团在 C9 位置。这种合成策略导致了 nemorosonol 的全合成,从 2-methyl-2-cyclopenten-1-one 分 12 步完成。
更新日期:2022-06-15
中文翻译:

PPAP、Nemorosonol 的全合成,使用串联迈克尔加成 - 分子内羟醛反应
已经建立了构建三环[4.3.1.0 3,7 ]癸烷骨架的策略,这是许多多环聚异戊二烯化酰基间苯三酚共有的骨架。关键步骤是串联迈克尔加成 - 与 3-ethoxy-1-phenyl-2-proyn-1-one 的分子内醛醇反应,得到在 C8 上具有苯甲酰基的三环[4.3.1.0 3,7 ]癸烷骨架位置和适当的氧官能团在 C9 位置。这种合成策略导致了 nemorosonol 的全合成,从 2-methyl-2-cyclopenten-1-one 分 12 步完成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号