当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The C-terminal head domain of Burkholderia pseudomallei BpaC has a striking hydrophilic core with an extensive solvent network
Molecular Microbiology ( IF 2.6 ) Pub Date : 2022-06-15 , DOI: 10.1111/mmi.14953 Andreas R Kiessling 1 , Sarah A Harris 2 , Kathleen M Weimer 1, 3 , Geoffrey Wells 4 , Adrian Goldman 1, 5
Molecular Microbiology ( IF 2.6 ) Pub Date : 2022-06-15 , DOI: 10.1111/mmi.14953 Andreas R Kiessling 1 , Sarah A Harris 2 , Kathleen M Weimer 1, 3 , Geoffrey Wells 4 , Adrian Goldman 1, 5
Affiliation
|
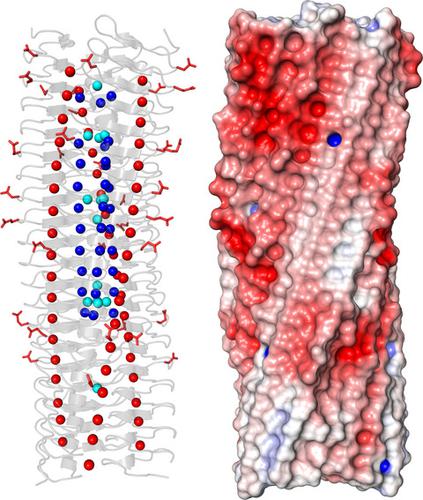
Gram-negative pathogens like Burkholderia pseudomallei use trimeric autotransporter adhesins such as BpaC as key molecules in their pathogenicity. Our 1.4 Å crystal structure of the membrane-proximal part of the BpaC head domain shows that the domain is exclusively made of left-handed parallel β-roll repeats. This, the largest such structure solved, has two unique features. First, the core, rather than being composed of the canonical hydrophobic Ile and Val, is made up primarily of the hydrophilic Thr and Asn, with two different solvent channels. Second, comparing BpaC to all other left-handed parallel β-roll structures showed that the position of the head domain in the protein correlates with the number and type of charged residues. In BpaC, only negatively charged residues face the solvent—in stark contrast to the primarily positive surface charge of the left-handed parallel β-roll “type” protein, YadA. We propose extending the definitions of these head domains to include the BpaC-like head domain as a separate subtype, based on its unusual sequence, position, and charge. We speculate that the function of left-handed parallel β-roll structures may differ depending on their position in the structure.
中文翻译:

Burkholderia pseudomallei BpaC 的 C 端头部结构域具有引人注目的亲水核心和广泛的溶剂网络
革兰氏阴性病原体如假马氏伯克霍尔德氏菌使用三聚体自转运体粘附素(如 BpaC)作为其致病性的关键分子。我们的 BpaC 头域近膜部分的 1.4 Å 晶体结构表明,该域完全由左手平行 β-roll 重复组成。这是解决的最大的此类结构,具有两个独特的特征。首先,核心不是由典型的疏水性 Ile 和 Val 组成,而是主要由亲水性 Thr 和 Asn 组成,具有两个不同的溶剂通道。其次,将 BpaC 与所有其他左手平行 β-roll 结构进行比较表明,蛋白质中头部结构域的位置与带电残基的数量和类型相关。在 BpaC 中,只有带负电的残基面对溶剂——与左手平行 β-roll “型”蛋白的主要表面正电荷形成鲜明对比,亚达。我们建议扩展这些头部域的定义,将 BpaC 样头部域作为一个单独的亚型,基于其不寻常的序列、位置和电荷。我们推测左手平行β-roll结构的功能可能会根据它们在结构中的位置而有所不同。
更新日期:2022-06-15
中文翻译:

Burkholderia pseudomallei BpaC 的 C 端头部结构域具有引人注目的亲水核心和广泛的溶剂网络
革兰氏阴性病原体如假马氏伯克霍尔德氏菌使用三聚体自转运体粘附素(如 BpaC)作为其致病性的关键分子。我们的 BpaC 头域近膜部分的 1.4 Å 晶体结构表明,该域完全由左手平行 β-roll 重复组成。这是解决的最大的此类结构,具有两个独特的特征。首先,核心不是由典型的疏水性 Ile 和 Val 组成,而是主要由亲水性 Thr 和 Asn 组成,具有两个不同的溶剂通道。其次,将 BpaC 与所有其他左手平行 β-roll 结构进行比较表明,蛋白质中头部结构域的位置与带电残基的数量和类型相关。在 BpaC 中,只有带负电的残基面对溶剂——与左手平行 β-roll “型”蛋白的主要表面正电荷形成鲜明对比,亚达。我们建议扩展这些头部域的定义,将 BpaC 样头部域作为一个单独的亚型,基于其不寻常的序列、位置和电荷。我们推测左手平行β-roll结构的功能可能会根据它们在结构中的位置而有所不同。










































 京公网安备 11010802027423号
京公网安备 11010802027423号