当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enzymatically Controlled Nanoflares for Specific Molecular Recognition and Biosensing
Analytical Chemistry ( IF 6.7 ) Pub Date : 2022-06-15 , DOI: 10.1021/acs.analchem.2c00166 Ya Zhao 1 , Jian Zhao 2 , Jingfang Zhang 3 , Yan Sun 1 , Lele Li 2 , Zhengping Li 3 , Mengyuan Li 3
Analytical Chemistry ( IF 6.7 ) Pub Date : 2022-06-15 , DOI: 10.1021/acs.analchem.2c00166 Ya Zhao 1 , Jian Zhao 2 , Jingfang Zhang 3 , Yan Sun 1 , Lele Li 2 , Zhengping Li 3 , Mengyuan Li 3
Affiliation
|
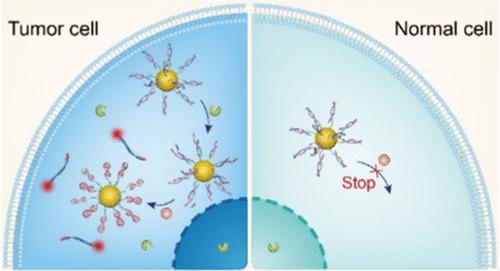
In situ sensing of physiological and pathological species in cancer cells is of great importance to unravel their molecular and cellular processes. However, the biosensing with conventional probes is often limited by the undesired on-target off-tumor interference. Here, we report a novel strategy to design enzymatically controlled nanoflares for sensing and imaging molecular targets in tumor cells. The triggerable nanoflare was designed via rational engineering of structure-switching aptamers with the incorporation of an enzyme-activatable site and further conjugation on gold nanoparticles. The nanoflare sensors did not respond to target molecules in normal cells, but they could be catalytically activated by specific enzymes in cancer cells, thereby enabling cancer-specific sensing and imaging in vitro and in vivo with improved tumor specificity. Considering that diverse aptamers were selected, we expect that this strategy would facilitate the precise detection of a broad range of targets in tumors and may promote the development of smart probes for cancer diagnosis.
中文翻译:

用于特定分子识别和生物传感的酶控纳米耀斑
癌细胞中生理和病理物种的原位传感对于揭示其分子和细胞过程非常重要。然而,传统探针的生物传感通常受到不希望的靶向肿瘤外干扰的限制。在这里,我们报告了一种新的策略来设计酶控制的纳米耀斑,用于传感和成像肿瘤细胞中的分子靶标。可触发的纳米耀斑是通过结构转换适体的合理工程设计的,其中结合了酶激活位点并进一步结合在金纳米颗粒上。纳米耀斑传感器对正常细胞中的靶分子没有反应,但它们可以被癌细胞中的特定酶催化激活,从而在体外和体内实现癌症特异性传感和成像,并提高肿瘤特异性。
更新日期:2022-06-15
中文翻译:

用于特定分子识别和生物传感的酶控纳米耀斑
癌细胞中生理和病理物种的原位传感对于揭示其分子和细胞过程非常重要。然而,传统探针的生物传感通常受到不希望的靶向肿瘤外干扰的限制。在这里,我们报告了一种新的策略来设计酶控制的纳米耀斑,用于传感和成像肿瘤细胞中的分子靶标。可触发的纳米耀斑是通过结构转换适体的合理工程设计的,其中结合了酶激活位点并进一步结合在金纳米颗粒上。纳米耀斑传感器对正常细胞中的靶分子没有反应,但它们可以被癌细胞中的特定酶催化激活,从而在体外和体内实现癌症特异性传感和成像,并提高肿瘤特异性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号