当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Introducing Co–O Moiety to Co–N–C Single-Atom Catalyst for Ethylbenzene Dehydrogenation
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-06-15 , DOI: 10.1021/acscatal.2c01873 Jiajia Shi 1, 2 , Yao Wei 2, 3, 4 , Dan Zhou 1 , Leilei Zhang 1 , Xiaofeng Yang 1 , Zhili Miao 1 , Haifeng Qi 1 , Shengxin Zhang 1, 2 , Anqi Li 1, 2 , Xiaoyan Liu 1 , Wensheng Yan 5 , Zheng Jiang 3, 4 , Aiqin Wang 1, 6 , Tao Zhang 1, 6
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-06-15 , DOI: 10.1021/acscatal.2c01873 Jiajia Shi 1, 2 , Yao Wei 2, 3, 4 , Dan Zhou 1 , Leilei Zhang 1 , Xiaofeng Yang 1 , Zhili Miao 1 , Haifeng Qi 1 , Shengxin Zhang 1, 2 , Anqi Li 1, 2 , Xiaoyan Liu 1 , Wensheng Yan 5 , Zheng Jiang 3, 4 , Aiqin Wang 1, 6 , Tao Zhang 1, 6
Affiliation
|
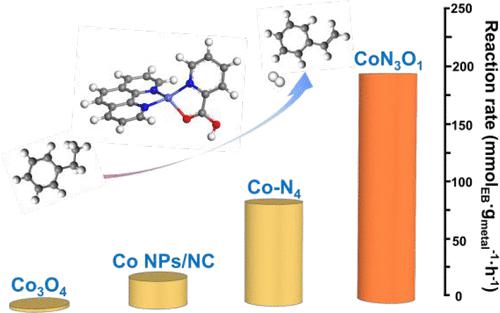
While single-atom catalysts (SACs) have been extensively studied as a type of high-atom-efficiency heterogeneous catalyst, their reaction stability under high temperature reductive atmosphere is yet to be addressed. In this work, we introduced a Co–O moiety to Co–N–C SACs by employing glutamic acid as both a N,O-bidentate ligand of Co(II) and a source for N-doped carbon. After undergoing pyrolysis in N2 at 900 °C, the complex transformed into the CoN3O1–OH2 structure and subsequently to the CoN3O1 structure upon being submitted to a high temperature reaction due to leaving out a weakly adsorbed water molecule, which was unambiguously identified by X-ray absorption spectroscopy combined with density functional theory calculations. The resulting CoN3O1 structure exhibited satisfactory activity and stability for ethylbenzene dehydrogenation at 550 °C, giving rise to a steady conversion rate of 4.7 mmolEB·gcat–1·h–1 and 192.9 mmolEB·gmetal–1·h–1, which was 74.2 times higher than that of Co3O4 and more than twice as high as those of Co NPs and O-free Co–N4 counterparts, manifesting the catalytically active role of the Co–O moiety. Intrinsic to alkane dehydrogenation, the initial activity decay was also observed for CoN3O1 SAC, which could be attributed to coking and loss of the ketonic carbonyl group on the N-doped carbon surface. The characterizations of the used catalyst after 30 h revealed that the CoN3O1 structure was well preserved without any aggregation of the Co species caused by the reduction of Co–N or C–O moieties, demonstrating the robustness of the CoN3O1 structure under a high-temperature reductive atmosphere. This work provides a route to the rational design of both active and stable SACs operating at high temperatures and in a reductive atmosphere.
中文翻译:

将 Co-O 基团引入 Co-N-C 单原子催化剂用于乙苯脱氢
虽然单原子催化剂(SACs)作为一种高原子效率的非均相催化剂已被广泛研究,但其在高温还原气氛下的反应稳定性仍有待解决。在这项工作中,我们通过使用谷氨酸作为 Co(II) 的 N,O-二齿配体和 N 掺杂碳的来源,将 Co-O 部分引入 Co-N-C SAC。在 900 °C在 N 2中进行热解后,络合物转变为 CoN 3 O 1 -OH 2结构,随后转变为 CoN 3 O 1由于遗漏了弱吸附的水分子,在进行高温反应后的结构,这通过 X 射线吸收光谱结合密度泛函理论计算明确地识别出来。得到的 CoN 3 O 1结构在 550 °C 下表现出令人满意的乙苯脱氢活性和稳定性,产生 4.7 mmol EB ·g cat –1 ·h –1和 192.9 mmol EB ·g metal –1 ·的稳定转化率。 h -1 ,是Co 3 O 4的74.2倍并且是 Co NPs 和无 O 的 Co-N 4对应物的两倍以上,表明 Co-O 部分的催化活性作用。对于烷烃脱氢,也观察到 CoN 3 O 1 SAC 的初始活性衰减,这可归因于 N 掺杂碳表面上的焦化和酮羰基的损失。30 小时后所用催化剂的表征表明,CoN 3 O 1结构保存完好,没有因 Co-N 或 C-O 部分的还原而导致 Co 物种聚集,证明了 CoN 3 O 1的稳健性高温还原气氛下的结构。这项工作为合理设计在高温和还原性气氛中运行的活性和稳定的 SAC 提供了一条途径。
更新日期:2022-06-15
中文翻译:

将 Co-O 基团引入 Co-N-C 单原子催化剂用于乙苯脱氢
虽然单原子催化剂(SACs)作为一种高原子效率的非均相催化剂已被广泛研究,但其在高温还原气氛下的反应稳定性仍有待解决。在这项工作中,我们通过使用谷氨酸作为 Co(II) 的 N,O-二齿配体和 N 掺杂碳的来源,将 Co-O 部分引入 Co-N-C SAC。在 900 °C在 N 2中进行热解后,络合物转变为 CoN 3 O 1 -OH 2结构,随后转变为 CoN 3 O 1由于遗漏了弱吸附的水分子,在进行高温反应后的结构,这通过 X 射线吸收光谱结合密度泛函理论计算明确地识别出来。得到的 CoN 3 O 1结构在 550 °C 下表现出令人满意的乙苯脱氢活性和稳定性,产生 4.7 mmol EB ·g cat –1 ·h –1和 192.9 mmol EB ·g metal –1 ·的稳定转化率。 h -1 ,是Co 3 O 4的74.2倍并且是 Co NPs 和无 O 的 Co-N 4对应物的两倍以上,表明 Co-O 部分的催化活性作用。对于烷烃脱氢,也观察到 CoN 3 O 1 SAC 的初始活性衰减,这可归因于 N 掺杂碳表面上的焦化和酮羰基的损失。30 小时后所用催化剂的表征表明,CoN 3 O 1结构保存完好,没有因 Co-N 或 C-O 部分的还原而导致 Co 物种聚集,证明了 CoN 3 O 1的稳健性高温还原气氛下的结构。这项工作为合理设计在高温和还原性气氛中运行的活性和稳定的 SAC 提供了一条途径。










































 京公网安备 11010802027423号
京公网安备 11010802027423号