当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Pentaketide Ansamycin Microansamycin H
Organic Letters ( IF 4.9 ) Pub Date : 2022-06-14 , DOI: 10.1021/acs.orglett.2c01760 Satapanawat Sittihan 1 , Somsak Ruchirawat 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2022-06-14 , DOI: 10.1021/acs.orglett.2c01760 Satapanawat Sittihan 1 , Somsak Ruchirawat 1, 2
Affiliation
|
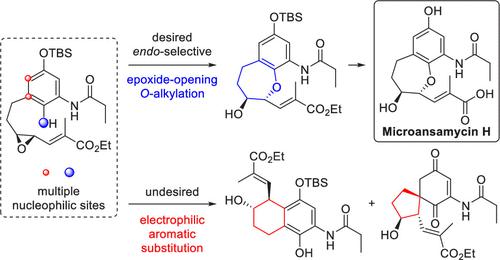
Herein, we report the first total synthesis of pentaketide ansamycin microansamycin H. Key to our success was the endo-selective epoxide-opening O-alkylation to construct the elusive seven-membered benzoxepane core. Due to the electron-rich disposition of the aromatic substrate, our pivotal transformation was hindered by competing electrophilic aromatic substitution at multiple C-based nucleophilic sites that generated kinetically favored products. Judicious choices of transition metal Lewis acid promoters biased toward the formation of the desired oxepane.
更新日期:2022-06-14











































 京公网安备 11010802027423号
京公网安备 11010802027423号