当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Production of pentyl valerate from γ-valerolactone, pentanol and H2 using Pd and Rh-based bifunctional catalysts
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2022-06-15 , DOI: 10.1039/d2re00121g Karla G. Martínez Figueredo 1 , Emanuel M. Virgilio 1 , Darío J. Segobia 1 , Nicolás M. Bertero 1
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2022-06-15 , DOI: 10.1039/d2re00121g Karla G. Martínez Figueredo 1 , Emanuel M. Virgilio 1 , Darío J. Segobia 1 , Nicolás M. Bertero 1
Affiliation
|
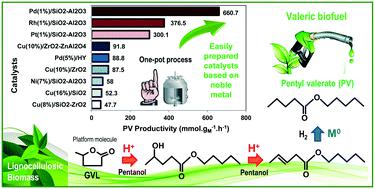
The production of pentyl valerate (PV) from γ-valerolactone (GVL) in the liquid phase in the presence of pentanol and H2 over Rh and Pd-based catalysts, using SiO2–Al2O3 (SA) as a support, was studied in this work. Both catalysts had metal loadings lower than 1 wt% and were prepared by incipient wetness impregnation using chlorinated metal precursors. The M0/SA catalysts exhibited a specific surface area and pore volume slightly lower than those of the SA support and the presence of metal nanoparticles with an average size of 5.6 nm for Rh/SA and 3.7 nm for Pd/SA. A total acid site density 10% lower than that of SA was determined for Rh/SA, whereas a value 45% higher was observed for Pd/SA. The relative strength of acid sites followed the pattern: Pd/SA > Rh/SA > SA with mainly Lewis acid sites in all the cases. Pd/SA showed a higher activity, selectivity to PV and carbon balance than Rh/SA. These results indicate that both a relatively high acid site density and smaller metal particles promote the conversion of adsorbed intermediates, reaching a GVL conversion of 81.1% and a PV yield of 70.1% over Pd/SA after 8 h. Therefore, PV productivity values 25% and 120% higher than the best reported value were reached over Rh/SA and Pd/SA, respectively, showing the potential of these catalysts to convert GVL into biofuels for transportation purposes. Finally, with Pd/SA, the apparent activation energy was equal to 25.6 kcal mol−1, the reaction order for H2 was about zero and a unitary reaction order for GVL was determined.
中文翻译:

使用 Pd 和 Rh 基双功能催化剂从 γ-戊内酯、戊醇和 H2 生产戊酸戊酯
使用 SiO 2 –Al 2 O 3 (SA) 作为载体,在戊醇和 H 2存在下,在 Rh 和 Pd 基催化剂上,从液相中的 γ-戊内酯 (GVL) 生产戊酸戊酯 (PV) ,在这项工作中进行了研究。两种催化剂的金属负载量均低于 1 wt%,并通过使用氯化金属前体的初湿浸渍制备。M 0/SA 催化剂的比表面积和孔体积略低于 SA 载体,并且存在平均尺寸为 Rh/SA 为 5.6 nm 和 Pd/SA 为 3.7 nm 的金属纳米颗粒。Rh/SA 的总酸位密度比 SA 低 10%,而 Pd/SA 的值高 45%。在所有情况下,酸性位点的相对强度遵循以下模式:Pd/SA > Rh/SA > SA,主要是路易斯酸性位点。Pd/SA 表现出比 Rh/SA 更高的活性、对 PV 的选择性和碳平衡。这些结果表明,相对较高的酸位密度和较小的金属颗粒都促进了吸附中间体的转化,在 8 小时后与 Pd/SA 相比,GVL 转化率为 81.1%,PV 产率为 70.1%。所以,在 Rh/SA 和 Pd/SA 上,PV 生产率值分别比报告的最佳值高 25% 和 120%,这表明这些催化剂具有将 GVL 转化为用于运输目的的生物燃料的潜力。最后,对于 Pd/SA,表观活化能等于 25.6 kcal mol-1,H 2的反应级数大约为零,并且确定了GVL的单一反应级数。
更新日期:2022-06-15
中文翻译:

使用 Pd 和 Rh 基双功能催化剂从 γ-戊内酯、戊醇和 H2 生产戊酸戊酯
使用 SiO 2 –Al 2 O 3 (SA) 作为载体,在戊醇和 H 2存在下,在 Rh 和 Pd 基催化剂上,从液相中的 γ-戊内酯 (GVL) 生产戊酸戊酯 (PV) ,在这项工作中进行了研究。两种催化剂的金属负载量均低于 1 wt%,并通过使用氯化金属前体的初湿浸渍制备。M 0/SA 催化剂的比表面积和孔体积略低于 SA 载体,并且存在平均尺寸为 Rh/SA 为 5.6 nm 和 Pd/SA 为 3.7 nm 的金属纳米颗粒。Rh/SA 的总酸位密度比 SA 低 10%,而 Pd/SA 的值高 45%。在所有情况下,酸性位点的相对强度遵循以下模式:Pd/SA > Rh/SA > SA,主要是路易斯酸性位点。Pd/SA 表现出比 Rh/SA 更高的活性、对 PV 的选择性和碳平衡。这些结果表明,相对较高的酸位密度和较小的金属颗粒都促进了吸附中间体的转化,在 8 小时后与 Pd/SA 相比,GVL 转化率为 81.1%,PV 产率为 70.1%。所以,在 Rh/SA 和 Pd/SA 上,PV 生产率值分别比报告的最佳值高 25% 和 120%,这表明这些催化剂具有将 GVL 转化为用于运输目的的生物燃料的潜力。最后,对于 Pd/SA,表观活化能等于 25.6 kcal mol-1,H 2的反应级数大约为零,并且确定了GVL的单一反应级数。











































 京公网安备 11010802027423号
京公网安备 11010802027423号