European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2022-06-13 , DOI: 10.1016/j.ejmech.2022.114505 Tsuyoshi Saitoh 1 , Mao Amezawa 2 , Jumpei Horiuchi 2 , Yasuyuki Nagumo 3 , Naoshi Yamamoto 3 , Noriki Kutsumura 4 , Ryuichiro Ohshita 2 , Akihisa Tokuda 5 , Yoko Irukayama-Tomobe 3 , Yasuhiro Ogawa 5 , Yukiko Ishikawa 3 , Emi Hasegawa 1 , Takeshi Sakurai 1 , Yasuo Uchida 6 , Tetsu Sato 6 , Hiroaki Gouda 7 , Ryuji Tanimura 8 , Masashi Yanagisawa 9 , Hiroshi Nagase 4
|
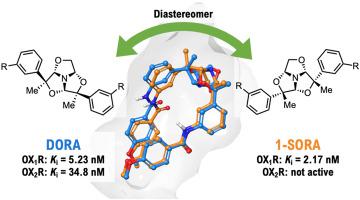
Structurally diverse small compounds are utilized to obtain hit compounds that have suitable pharmacophores in appropriate three-dimensional conformations for the target drug receptors. We have focused on the 1,3,5-trioxazatriquinane skeleton, which has a rigid bowl-like structure enabling the diverse orientation of side chain units, leading to a novel small-scale focused library based on the skeleton. In the library screening for the orexin receptor, some of the compounds showed orexin receptor antagonistic activity with a high hit rate of 7%. By optimizing the hit compounds, we discovered a potent dual orexin receptor antagonist, 38b, and a selective orexin 1 receptor antagonist, 41b carrying the same plane structure. Both compounds showed reasonable brain permeability and beneficial effects when administered intraperitoneally to wild-type mice. Docking simulations of their eutomers, (−)-38b and (+)-41b, with orexin receptors suggested that the interaction between the 1,3,5-trioxazatriquinane core structure and the hydrophobic subpocket in orexin receptors enables a U-shape structure, which causes tight van der Waals interactions with the receptors similar to SB-334867, a selective orexin 1 receptor antagonist. These results indicate that the library approach utilizing the 1,3,5-trioxazatriquinanes bearing multiple effective residues (TriMERs) might be useful for the hit discovery process targeting not only opioid and orexin receptors but other G-protein coupled receptors.
中文翻译:

使用带有多个有效残基 (TriMER) 库的 1,3,5-三恶唑三喹烷发现新型食欲素受体拮抗剂
利用结构多样的小化合物来获得具有适合靶药物受体的适当三维构象的药效团的命中化合物。我们专注于 1,3,5-trioxazatriquinane 骨架,它具有刚性的碗状结构,能够实现侧链单元的不同方向,从而形成了一个基于骨架的新型小型聚焦库。在食欲素受体文库筛选中,部分化合物表现出食欲素受体拮抗活性,命中率高达7%。通过优化命中化合物,我们发现了一种有效的双食欲素受体拮抗剂38b和一种选择性食欲素 1 受体拮抗剂41b承载相同的平面结构。当对野生型小鼠进行腹膜内给药时,两种化合物均显示出合理的脑渗透性和有益效果。他们的eutomers的对接模拟,(-)- 38b和(+)- 41b,食欲素受体表明 1,3,5-三恶杂三喹烷核心结构和食欲素受体中的疏水亚袋之间的相互作用形成了 U 形结构,这导致与受体的紧密范德华相互作用,类似于 SB-334867,a选择性食欲素1受体拮抗剂。这些结果表明,利用带有多个有效残基 (TriMER) 的 1,3,5-三恶唑三喹烷的文库方法可能对不仅针对阿片类药物和食欲素受体而且针对其他 G 蛋白偶联受体的命中发现过程有用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号