当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iridium-Catalyzed Intermolecular Asymmetric Allylic Amination with Pyridones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-06-14 , DOI: 10.1002/adsc.202200347 Hang-Fei Tu 1 , Yu-Han Nie 1 , Chao Zheng 1 , Shu-Li You 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-06-14 , DOI: 10.1002/adsc.202200347 Hang-Fei Tu 1 , Yu-Han Nie 1 , Chao Zheng 1 , Shu-Li You 1
Affiliation
|
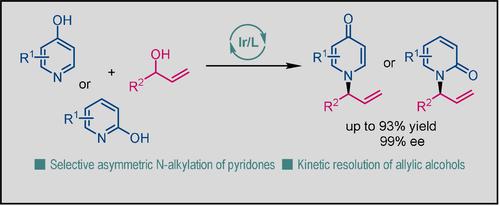
We report herein a catalytic synthetic method for enantioenriched N-substituted pyridones. By employing chiral iridium catalyst generated from the Carreira [P/olefin] ligand, intermolecular asymmetric allylic amination of allyl alcohols with pyridones proceeded smoothly with excellent chemo-, regio- and enantioselectivities (>19:1 N/O, >19:1 b/l and ≥89% ee). The reaction was a kinetic resolution process under mild conditions and displayed a broad substrate scope for both pyridones and allylic alcohols. The potential of this method was demonstrated by a gram-scale reaction with only 0.6 mol% catalyst loading and diverse transformations of the amination products to useful skeletons.
中文翻译:

铱催化的吡啶酮分子间不对称烯丙基胺化
我们在此报告了一种对映体富集的N-取代吡啶酮的催化合成方法。通过使用由 Carreira [P/烯烃] 配体生成的手性铱催化剂,烯丙醇与吡啶酮的分子间不对称烯丙基胺化顺利进行,具有优异的化学选择性、区域选择性和对映选择性 (>19:1 N / O , >19:1 b /l 和≥89% ee)。该反应是在温和条件下的动力学拆分过程,并且对吡啶酮和烯丙醇都显示出广泛的底物范围。这种方法的潜力通过仅 0.6 mol% 催化剂负载的克级反应和胺化产物向有用骨架的多种转化得到证明。
更新日期:2022-06-14
中文翻译:

铱催化的吡啶酮分子间不对称烯丙基胺化
我们在此报告了一种对映体富集的N-取代吡啶酮的催化合成方法。通过使用由 Carreira [P/烯烃] 配体生成的手性铱催化剂,烯丙醇与吡啶酮的分子间不对称烯丙基胺化顺利进行,具有优异的化学选择性、区域选择性和对映选择性 (>19:1 N / O , >19:1 b /l 和≥89% ee)。该反应是在温和条件下的动力学拆分过程,并且对吡啶酮和烯丙醇都显示出广泛的底物范围。这种方法的潜力通过仅 0.6 mol% 催化剂负载的克级反应和胺化产物向有用骨架的多种转化得到证明。










































 京公网安备 11010802027423号
京公网安备 11010802027423号