当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic and structural studies of the reaction of Escherichia coli dihydrodipicolinate synthase with (S)-2-bromopropionate
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2022-06-14 , DOI: 10.1107/s2059798322005125 Lilian Chooback 1 , Leonard N Thomas 2 , Nathan Blythe 1 , William Karsten 2
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2022-06-14 , DOI: 10.1107/s2059798322005125 Lilian Chooback 1 , Leonard N Thomas 2 , Nathan Blythe 1 , William Karsten 2
Affiliation
|
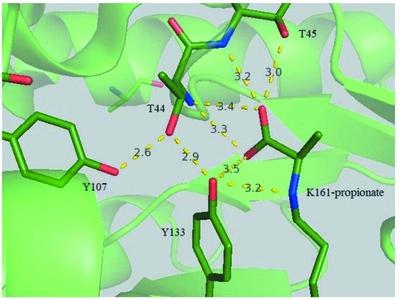
Dihydrodipicolinate synthase (DHDPS) catalyzes the first committed step in the lysine-biosynthetic pathway converting pyruvate and l-aspartate-β-semialdehyde to dihydrodipicolinate. Kinetic studies indicate that the pyruvate analog (S)-2-bromopropionate inactivates the enzyme in a pseudo-first-order process. An initial velocity pattern indicates that (S)-2-bromopropionate is a competitive inhibitor versus pyruvate, with an inhibition constant of about 8 mM. Crystals of DHDPS complexed with (S)-2-bromopropionate formed in a solution consisting of 50 mM HEPES pH 7.5, 18% polyethylene glycol 3350, 8 mM spermidine, 0.2 M sodium tartrate and 5.0 mg ml−1 DHDPS. The crystals diffracted to 2.15 Å resolution and belonged to space group P1. The crystal structure confirms the displacement of bromine and the formation of a covalent attachment between propionate and Lys161 at the active site of the enzyme. Lys161 is the active-site nucleophile that attacks the carbonyl C atom of pyruvate and subsequently generates an imine adduct in the first half-reaction of the ping-pong enzymatic reaction. A comparison of the crystal structures of DHDPS complexed with pyruvate or (S)-2-bromopropionate indicates the covalent adduct formed from (S)-2-bromopropionate leads to a rotation of about 180° of the β–δ C atoms of Lys61 that aligns the covalently bound propionate fairly closely with the imine adduct formed with pyruvate.
中文翻译:

大肠杆菌二氢吡啶二羧酸合酶与(S)-2-溴丙酸反应的动力学和结构研究
二氢吡啶二羧酸合成酶 (DHDPS) 催化赖氨酸生物合成途径中的第一个关键步骤,将丙酮酸和L-天冬氨酸-β-半醛转化为二氢吡啶二羧酸。动力学研究表明,丙酮酸类似物 ( S )-2-溴丙酸以伪一级过程使酶失活。初始速度模式表明 ( S )-2-溴丙酸与丙酮酸相比是一种竞争性抑制剂,抑制常数约为 8 m M。 DHDPS 与 ( S )-2-溴丙酸盐复合的晶体在由 50 m M HEPES pH 7.5、18% 聚乙二醇 3350、8 m M亚精胺、0.2 M酒石酸钠和 5.0 mg ml -1 DHDPS 组成的溶液中形成。晶体衍射分辨率为 2.15 Å,属于空间群P 1。晶体结构证实了溴的置换以及丙酸和 Lys161 在酶活性位点之间形成共价连接。 Lys161 是活性位点亲核试剂,可攻击丙酮酸的羰基 C 原子,随后在乒乓酶反应的第一个半反应中生成亚胺加合物。与丙酮酸或 ( S )-2-溴丙酸复合的 DHDPS 晶体结构的比较表明,由 ( S )-2-溴丙酸形成的共价加合物导致 Lys61 的 β-δ C 原子旋转约 180°,使共价结合的丙酸盐与丙酮酸盐形成的亚胺加合物相当紧密地排列。
更新日期:2022-06-14
中文翻译:

大肠杆菌二氢吡啶二羧酸合酶与(S)-2-溴丙酸反应的动力学和结构研究
二氢吡啶二羧酸合成酶 (DHDPS) 催化赖氨酸生物合成途径中的第一个关键步骤,将丙酮酸和L-天冬氨酸-β-半醛转化为二氢吡啶二羧酸。动力学研究表明,丙酮酸类似物 ( S )-2-溴丙酸以伪一级过程使酶失活。初始速度模式表明 ( S )-2-溴丙酸与丙酮酸相比是一种竞争性抑制剂,抑制常数约为 8 m M。 DHDPS 与 ( S )-2-溴丙酸盐复合的晶体在由 50 m M HEPES pH 7.5、18% 聚乙二醇 3350、8 m M亚精胺、0.2 M酒石酸钠和 5.0 mg ml -1 DHDPS 组成的溶液中形成。晶体衍射分辨率为 2.15 Å,属于空间群P 1。晶体结构证实了溴的置换以及丙酸和 Lys161 在酶活性位点之间形成共价连接。 Lys161 是活性位点亲核试剂,可攻击丙酮酸的羰基 C 原子,随后在乒乓酶反应的第一个半反应中生成亚胺加合物。与丙酮酸或 ( S )-2-溴丙酸复合的 DHDPS 晶体结构的比较表明,由 ( S )-2-溴丙酸形成的共价加合物导致 Lys61 的 β-δ C 原子旋转约 180°,使共价结合的丙酸盐与丙酮酸盐形成的亚胺加合物相当紧密地排列。











































 京公网安备 11010802027423号
京公网安备 11010802027423号