当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biochemical and structural insights into an unusual, alkali-metal-independent S-adenosyl-l-homocysteine hydrolase from Synechocystis sp. PCC 6803
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2022-06-14 , DOI: 10.1107/s2059798322005605 Piotr H Malecki 1 , Barbara Imiolczyk 1 , Jakub Barciszewski 2 , Justyna Czyrko-Horczak 3 , Joanna Sliwiak 2 , Magdalena Gawel 1 , Katarzyna Wozniak 1 , Mariusz Jaskolski 4 , Krzysztof Brzezinski 1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2022-06-14 , DOI: 10.1107/s2059798322005605 Piotr H Malecki 1 , Barbara Imiolczyk 1 , Jakub Barciszewski 2 , Justyna Czyrko-Horczak 3 , Joanna Sliwiak 2 , Magdalena Gawel 1 , Katarzyna Wozniak 1 , Mariusz Jaskolski 4 , Krzysztof Brzezinski 1
Affiliation
|
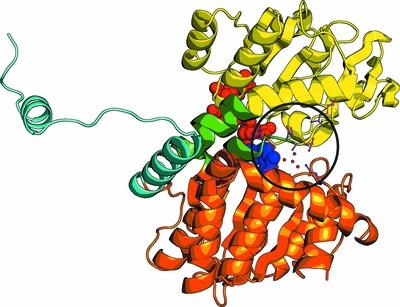
The mesophilic cyanobacterium Synechocystis sp. PCC 6803 encodes an S-adenosyl-l-homocysteine hydrolase (SAHase) of archaeal origin in its genome. SAHases are essential enzymes involved in the regulation of cellular S-adenosyl-l-methionine (SAM)-dependent methylation reactions. They are usually active as homotetramers or, less commonly, as homodimers. A SAHase subunit is composed of two major domains: a cofactor (NAD+)-binding domain and a substrate (S-adenosyl-l-homocysteine)-binding domain. These are connected by a hinge element that is also a coordination site for an alkali-metal cation that influences domain movement during the catalytic cycle. Typically, the highest activity and strongest substrate binding of bacterial SAHases are observed in the presence of K+ ions. The SAHase from Synechocystis (SynSAHase) is an exception in this respect. Enzymatic and isothermal titration calorimetry studies demonstrated that in contrast to K+-dependent SAHases, the activity and ligand binding of SynSAHase are not affected by the presence of any particular alkali ion. Moreover, in contrast to other SAHases, the cyanobacterial enzyme is in an equilibrium of two distinct oligomeric states corresponding to its dimeric and tetrameric forms in solution. To explain these phenomena, crystal structures of SynSAHase were determined for the enzyme crystallized in the presence of adenosine (a reaction byproduct or substrate) and sodium or rubidium cations. The structural data confirm that while SynSAHase shares common structural features with other SAHases, no alkali metal is coordinated by the cyanobacterial enzyme as a result of a different organization of the macromolecular environment of the site that is normally supposed to coordinate the metal cation. This inspired the generation of SynSAHase mutants that bind alkali-metal cations analogously to K+-dependent SAHases, as confirmed by crystallographic studies. Structural comparisons of the crystal structure of SynSAHase with other experimental models of SAHases suggest a possible explanation for the occurrence of the cyanobacterial enzyme in the tetrameric state. On the other hand, the reason for the existence of SynSAHase in the dimeric state in solution remains elusive.
中文翻译:

来自集胞藻属的一种不寻常的、不依赖碱金属的 S-腺苷-l-同型半胱氨酸水解酶的生化和结构见解。PCC 6803
嗜温蓝藻Synechocystis sp。PCC 6803 在其基因组中编码古细菌来源的S-腺苷-1-同型半胱氨酸水解酶 (SAHase)。SAHases 是参与调节细胞S-腺苷-1-甲硫氨酸 (SAM) 依赖性甲基化反应的必需酶。它们通常作为同型四聚体或不太常见的同型二聚体具有活性。SAHase 亚基由两个主要结构域组成:辅因子 (NAD + )-结合结构域和底物 ( S -adenosyl -l-同型半胱氨酸)-结合域。它们通过铰链元件连接,该铰链元件也是碱金属阳离子的配位点,在催化循环期间影响域运动。通常,在存在 K +离子的情况下观察到细菌 SAHase 的最高活性和最强的底物结合。来自集胞藻属的 SAHase ( SynSAHase) 在这方面是一个例外。酶促和等温滴定量热研究表明,与 K +依赖型 SAHase,SynSAHase 的活性和配体结合不受任何特定碱金属离子存在的影响。此外,与其他 SAHase 相比,蓝藻酶处于两种不同寡聚状态的平衡状态,对应于其在溶液中的二聚体和四聚体形式。为了解释这些现象,SynSAHase 的晶体结构是针对在腺苷(反应副产物或底物)和钠或铷阳离子存在下结晶的酶确定的。结构数据证实,虽然 SynSAHase 与其他 SAHase 具有共同的结构特征,但由于通常认为与金属阳离子配位的位点的大分子环境的不同组织,蓝藻酶没有配位碱金属。+ -依赖性SAHases,如晶体学研究所证实。SynSAHase 的晶体结构与其他 SAHases 实验模型的结构比较表明了蓝藻酶以四聚体状态出现的可能解释。另一方面,SynSAHase 在溶液中以二聚体状态存在的原因仍然难以捉摸。
更新日期:2022-06-14
中文翻译:

来自集胞藻属的一种不寻常的、不依赖碱金属的 S-腺苷-l-同型半胱氨酸水解酶的生化和结构见解。PCC 6803
嗜温蓝藻Synechocystis sp。PCC 6803 在其基因组中编码古细菌来源的S-腺苷-1-同型半胱氨酸水解酶 (SAHase)。SAHases 是参与调节细胞S-腺苷-1-甲硫氨酸 (SAM) 依赖性甲基化反应的必需酶。它们通常作为同型四聚体或不太常见的同型二聚体具有活性。SAHase 亚基由两个主要结构域组成:辅因子 (NAD + )-结合结构域和底物 ( S -adenosyl -l-同型半胱氨酸)-结合域。它们通过铰链元件连接,该铰链元件也是碱金属阳离子的配位点,在催化循环期间影响域运动。通常,在存在 K +离子的情况下观察到细菌 SAHase 的最高活性和最强的底物结合。来自集胞藻属的 SAHase ( SynSAHase) 在这方面是一个例外。酶促和等温滴定量热研究表明,与 K +依赖型 SAHase,SynSAHase 的活性和配体结合不受任何特定碱金属离子存在的影响。此外,与其他 SAHase 相比,蓝藻酶处于两种不同寡聚状态的平衡状态,对应于其在溶液中的二聚体和四聚体形式。为了解释这些现象,SynSAHase 的晶体结构是针对在腺苷(反应副产物或底物)和钠或铷阳离子存在下结晶的酶确定的。结构数据证实,虽然 SynSAHase 与其他 SAHase 具有共同的结构特征,但由于通常认为与金属阳离子配位的位点的大分子环境的不同组织,蓝藻酶没有配位碱金属。+ -依赖性SAHases,如晶体学研究所证实。SynSAHase 的晶体结构与其他 SAHases 实验模型的结构比较表明了蓝藻酶以四聚体状态出现的可能解释。另一方面,SynSAHase 在溶液中以二聚体状态存在的原因仍然难以捉摸。











































 京公网安备 11010802027423号
京公网安备 11010802027423号