当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural basis of ligand recognition and transport by Sfh2, a yeast phosphatidylinositol transfer protein of the Sec14 superfamily
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2022-06-14 , DOI: 10.1107/s2059798322005666 Lin Chen 1 , Lingchen Tan 1 , Young Jun Im 1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2022-06-14 , DOI: 10.1107/s2059798322005666 Lin Chen 1 , Lingchen Tan 1 , Young Jun Im 1
Affiliation
|
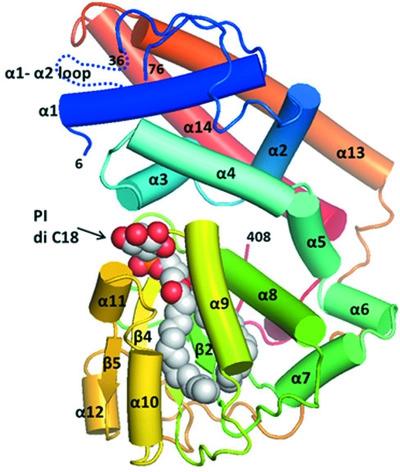
Sec14-like phosphatidylinositol transfer proteins (PITPs) are involved in lipid metabolism and phosphatidylinositol 4-phosphate signaling by transporting phosphatidylinositol (PI) and a secondary ligand between the organellar membranes in eukaryotes. Yeast Sfh2 is a PITP that transfers PI and squalene without phosphatidylcholine transfer activity. To investigate the structural determinants for ligand specificity and transport in Sfh2, crystal structures of Sfh2 in complex with PI and squalene were determined at 1.5 and 2.4 Å resolution, respectively. The inositol head group of PI is recognized by highly conserved residues around the pocket entrance. The acyl chains of PI bind into a large hydrophobic cavity. Squalene is accommodated in the bottom of the cavity entirely by hydrophobic interactions. The binding of PI and squalene are mutually exclusive due to their overlapping binding sites, correlating with the role in lipid exchange. The binding mode of PI is well conserved in Sfh family proteins. However, squalene binding is unique to the Sfh2 homolog due to the specific hydrophobic residues forming a shape-complementary binding pocket. Recombinant apo Sfh2 forms a homodimer in vitro by the hydrophobic interaction of the gating α10–α11 helices in an open conformation. Ligand binding closes the lid and dissociates the dimer into monomers. This study reveals the structural determinants for the recognition of the conserved PI and a secondary ligand, squalene, and provides implications for the lipid-transfer function of Sfh2.
中文翻译:

Sfh2配体识别和转运的结构基础,Sec14超家族的酵母磷脂酰肌醇转移蛋白
Sec14 样磷脂酰肌醇转移蛋白 (PITP) 通过在真核生物的细胞器膜之间转运磷脂酰肌醇 (PI) 和二级配体参与脂质代谢和 4-磷酸磷脂酰肌醇信号传导。Yeast Sfh2 是一种 PITP,可在没有磷脂酰胆碱转移活性的情况下转移 PI 和角鲨烯。为了研究 Sfh2 中配体特异性和转运的结构决定因素,分别以 1.5 和 2.4 Å 的分辨率测定了 Sfh2 与 PI 和角鲨烯复合的晶体结构。PI 的肌醇头组被口袋入口周围高度保守的残基识别。PI 的酰基链结合成一个大的疏水空腔。角鲨烯完全通过疏水相互作用容纳在腔的底部。PI 和角鲨烯的结合是相互排斥的,因为它们的结合位点重叠,与脂质交换中的作用相关。PI 的结合模式在 Sfh 家族蛋白中非常保守。然而,角鲨烯结合是 Sfh2 同系物所独有的,因为特定的疏水残基形成了形状互补的结合袋。重组 apo Sfh2 形成同型二聚体在体外通过开放构象中的门控α10-α11螺旋的疏水相互作用。配体结合关闭盖子并将二聚体解离成单体。本研究揭示了识别保守 PI 和二级配体角鲨烯的结构决定因素,并为 Sfh2 的脂质转移功能提供了启示。
更新日期:2022-06-14
中文翻译:

Sfh2配体识别和转运的结构基础,Sec14超家族的酵母磷脂酰肌醇转移蛋白
Sec14 样磷脂酰肌醇转移蛋白 (PITP) 通过在真核生物的细胞器膜之间转运磷脂酰肌醇 (PI) 和二级配体参与脂质代谢和 4-磷酸磷脂酰肌醇信号传导。Yeast Sfh2 是一种 PITP,可在没有磷脂酰胆碱转移活性的情况下转移 PI 和角鲨烯。为了研究 Sfh2 中配体特异性和转运的结构决定因素,分别以 1.5 和 2.4 Å 的分辨率测定了 Sfh2 与 PI 和角鲨烯复合的晶体结构。PI 的肌醇头组被口袋入口周围高度保守的残基识别。PI 的酰基链结合成一个大的疏水空腔。角鲨烯完全通过疏水相互作用容纳在腔的底部。PI 和角鲨烯的结合是相互排斥的,因为它们的结合位点重叠,与脂质交换中的作用相关。PI 的结合模式在 Sfh 家族蛋白中非常保守。然而,角鲨烯结合是 Sfh2 同系物所独有的,因为特定的疏水残基形成了形状互补的结合袋。重组 apo Sfh2 形成同型二聚体在体外通过开放构象中的门控α10-α11螺旋的疏水相互作用。配体结合关闭盖子并将二聚体解离成单体。本研究揭示了识别保守 PI 和二级配体角鲨烯的结构决定因素,并为 Sfh2 的脂质转移功能提供了启示。











































 京公网安备 11010802027423号
京公网安备 11010802027423号