当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-catalysed diversification of phosphine ligands by formal substitution at phosphorus
Chemical Science ( IF 7.6 ) Pub Date : 2022-06-14 , DOI: 10.1039/d2sc02496a Sven Roediger 1 , Sebastian U Leutenegger 1 , Bill Morandi 1
Chemical Science ( IF 7.6 ) Pub Date : 2022-06-14 , DOI: 10.1039/d2sc02496a Sven Roediger 1 , Sebastian U Leutenegger 1 , Bill Morandi 1
Affiliation
|
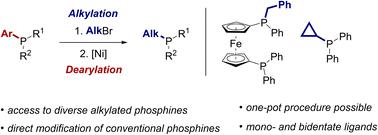
We report a diversification strategy that enables the direct substituent exchange of tertiary phosphines. Alkylated phosphonium salts, prepared by standard alkylation of phosphines, are selectively dearylated in a nickel-catalysed process to access alkylphosphine products via a formal substitution at the phosphorus center. The reaction can be used to introduce a wide range of alkyl substituents into both mono- and bisphosphines. We also show that the alkylation and dearylation steps can be conducted in a one-pot sequence, enabling accelerated access to derivatives of the parent ligand. The phosphine products of the reaction are converted in situ to air-stable borane adducts for isolation, and versatile derivatisation reactions of these adducts are demonstrated.
中文翻译:

镍催化磷配体的形式取代磷配体多样化
我们报告了一种多样化策略,可以实现叔膦的直接取代基交换。通过膦的标准烷基化制备的烷基化鏻盐在镍催化工艺中选择性脱芳基,通过磷中心的形式取代获得烷基膦产物。该反应可用于将范围广泛的烷基取代基引入单膦和双膦。我们还表明,烷基化和去芳基化步骤可以在一锅序列中进行,从而能够加速获得母配体的衍生物。该反应的膦产物原位转化为空气稳定的硼烷加合物以进行分离,并证明了这些加合物的通用衍生反应。
更新日期:2022-06-14
中文翻译:

镍催化磷配体的形式取代磷配体多样化
我们报告了一种多样化策略,可以实现叔膦的直接取代基交换。通过膦的标准烷基化制备的烷基化鏻盐在镍催化工艺中选择性脱芳基,通过磷中心的形式取代获得烷基膦产物。该反应可用于将范围广泛的烷基取代基引入单膦和双膦。我们还表明,烷基化和去芳基化步骤可以在一锅序列中进行,从而能够加速获得母配体的衍生物。该反应的膦产物原位转化为空气稳定的硼烷加合物以进行分离,并证明了这些加合物的通用衍生反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号