当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total synthesis of thioamycolamide A using diastereoselective sulfa-Michael addition as the key step
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-06-14 , DOI: 10.1039/d2qo00747a Yi Xiao 1 , Yangyang Jiang 1 , Chao Xu 2 , Pratanphorn Nakliang 3 , Sanghee Yoon 3 , Sun Choi 3 , Yian Guo 1, 2 , Tao Ye 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-06-14 , DOI: 10.1039/d2qo00747a Yi Xiao 1 , Yangyang Jiang 1 , Chao Xu 2 , Pratanphorn Nakliang 3 , Sanghee Yoon 3 , Sun Choi 3 , Yian Guo 1, 2 , Tao Ye 1
Affiliation
|
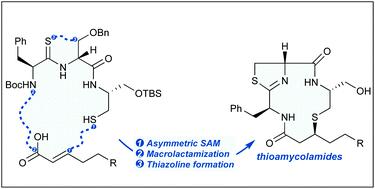
The stereocontrolled total synthesis of the antitumor natural product thioamycolamide A has been accomplished in 14 longest linear steps and an overall yield of 19.1%. The central feature of our convergent route to this family of novel macrocyclic natural products is the preparation of the β-alkylthio amide subunit through an auxiliary-controlled, diastereoselective sulfa-Michael addition.
中文翻译:

以非对映选择性磺胺-迈克尔加成为关键步骤全合成硫代戊酰胺A
抗肿瘤天然产物硫代淀粉酰胺 A 的立体控制全合成通过 14 个最长的线性步骤完成,总产率为 19.1%。我们通往这一新型大环天然产物家族的收敛路线的核心特征是通过辅助控制的非对映选择性磺胺-迈克尔加成制备 β-烷硫基酰胺亚基。
更新日期:2022-06-14
中文翻译:

以非对映选择性磺胺-迈克尔加成为关键步骤全合成硫代戊酰胺A
抗肿瘤天然产物硫代淀粉酰胺 A 的立体控制全合成通过 14 个最长的线性步骤完成,总产率为 19.1%。我们通往这一新型大环天然产物家族的收敛路线的核心特征是通过辅助控制的非对映选择性磺胺-迈克尔加成制备 β-烷硫基酰胺亚基。











































 京公网安备 11010802027423号
京公网安备 11010802027423号