当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
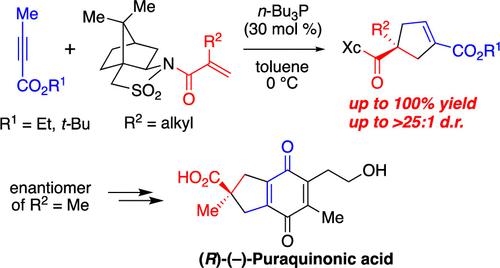
Asymmetric Synthesis of Cyclopentene Compounds Containing All-Carbon Quaternary Stereocenters by (3 + 2) Cycloaddition and Its Application in the Formal Synthesis of (R)-(−)-Puraquinonic Acid
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-14 , DOI: 10.1021/acs.joc.2c00753 Miho Oga 1 , Yusei Takamatsu 1 , Akihiro Ogura 1 , Ken-Ichi Takao 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-14 , DOI: 10.1021/acs.joc.2c00753 Miho Oga 1 , Yusei Takamatsu 1 , Akihiro Ogura 1 , Ken-Ichi Takao 1
Affiliation
|
A highly stereoselective (3 + 2) cycloaddition for the asymmetric synthesis of versatile cyclopentene compounds containing all-carbon quaternary stereocenters was developed. The phosphine-catalyzed reactions of alkynoates with α-alkylated electron-deficient alkenes bearing Oppolzer’s camphorsultam showed high to excellent diastereoselectivities and perfect regioselectivities. The usefulness of this reaction was demonstrated in the concise formal synthesis of (R)-(−)-puraquinonic acid.
中文翻译:

(3+2)环加成不对称合成全碳四元立体中心环戊烯化合物及其在(R)-(-)-嘌呤酸正式合成中的应用
开发了一种高度立体选择性 (3 + 2) 环加成,用于不对称合成含有全碳四元立体中心的通用环戊烯化合物。炔酸酯与带有 Oppolzer 樟脑磺胺的 α-烷基化缺电子烯烃的膦催化反应显示出高至优异的非对映选择性和完美的区域选择性。( R )-(-)-puraquinonic 酸的简明形式合成证明了该反应的有用性。
更新日期:2022-06-14
中文翻译:

(3+2)环加成不对称合成全碳四元立体中心环戊烯化合物及其在(R)-(-)-嘌呤酸正式合成中的应用
开发了一种高度立体选择性 (3 + 2) 环加成,用于不对称合成含有全碳四元立体中心的通用环戊烯化合物。炔酸酯与带有 Oppolzer 樟脑磺胺的 α-烷基化缺电子烯烃的膦催化反应显示出高至优异的非对映选择性和完美的区域选择性。( R )-(-)-puraquinonic 酸的简明形式合成证明了该反应的有用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号