当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Atomic-Scale Insights into Comparative Mechanisms and Kinetics of Na–S and Li–S Batteries
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-06-14 , DOI: 10.1021/acscatal.2c01174 Md Shahriar Nahian 1 , Rahul Jayan 1 , Md Mahbubul Islam 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-06-14 , DOI: 10.1021/acscatal.2c01174 Md Shahriar Nahian 1 , Rahul Jayan 1 , Md Mahbubul Islam 1
Affiliation
|
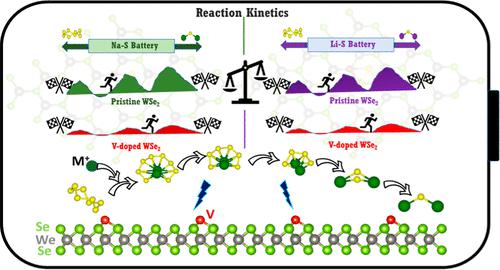
The Na–S and Li–S batteries are in the forefront to supplant ubiquitously used lithium-ion batteries. The understanding of mechanistic differences between Na–S and Li–S is critical to enable the inter-transfer of developed technologies toward designing high-performance cathode materials. The anchoring materials (AMs) are required to overcome the performance-limiting factors such as sluggish kinetics of metal polysulfides’ (M2Sn, M = Na and Li, n = 1–8) conversion reactions and their dissolution into electrolytes. This study undertakes the challenges to critically understand the role of AMs on the polysulfide chemistry in both the batteries. We employ first-principles density functional theory simulations to comprehensively examine the adsorption mechanisms of M2Sn and the kinetics of sulfur reduction reactions (SRRs) and the catalytic decomposition of short-chain polysulfides across Na–S and Li–S batteries on pristine and vanadium (V) single-atom catalyst embedded WSe2 (V@WSe2) substrates. We found that pristine WSe2 cannot immobilize the higher-order M2Sn; however, V@WSe2 endows adequate binding energies to trap the higher-order M2Sn. The degree of M2Sn adsorption strengths and the effectiveness of the V@WSe2 varies between Na–S and Li–S systems. We elucidate the underlying mechanistic details with the aid of charge transfer, bond strength, and density of state analysis. Importantly, our simulations reveal that, in V@WSe2, the rate-limiting step of the SRR is kinetically faster in Li–S, whereas the oxidative decomposition of the discharge end product M2S exhibits accelerated kinetics in Na–S batteries. These findings are pivotal to understand the role of AMs in the design of cathode materials for addressing the performance-limiting factors in Na–S and Li–S batteries, in particular, and metal–sulfur batteries, in general.
中文翻译:

Na-S 和 Li-S 电池的比较机理和动力学的原子尺度洞察
Na-S 和 Li-S 电池在取代普遍使用的锂离子电池方面处于领先地位。了解 Na-S 和 Li-S 之间的机械差异对于实现已开发技术向设计高性能正极材料的相互转移至关重要。锚固材料 (AMs) 需要克服性能限制因素,例如金属多硫化物 (M 2 S n , M = Na 和 Li, n= 1-8) 转化反应及其溶解到电解质中。本研究面临挑战,以批判性地了解 AM 在两种电池中的多硫化物化学中的作用。我们采用第一性原理密度泛函理论模拟来全面检查 M 2 Sn的吸附机制、硫还原反应 ( SRR ) 的动力学以及短链多硫化物在 Na-S 和 Li-S 电池上的催化分解。和钒 (V) 单原子催化剂嵌入 WSe 2 (V@WSe 2 ) 基板。我们发现原始WSe 2不能固定高阶M 2 S n;然而,V@WSe 2赋予足够的结合能来捕获高阶 M 2 S n。M 2 S n吸附强度的程度和 V@WSe 2的有效性在 Na-S 和 Li-S 系统之间有所不同。我们借助电荷转移、键强度和状态密度分析阐明了潜在的机械细节。重要的是,我们的模拟表明,在 V@WSe 2中,SRR 的限速步骤在 Li-S 中动力学更快,而放电终产物 M 2的氧化分解S 在 Na-S 电池中表现出加速动力学。这些发现对于理解 AM 在正极材料设计中的作用至关重要,以解决钠硫和锂硫电池(特别是金属硫电池)中的性能限制因素。
更新日期:2022-06-14
中文翻译:

Na-S 和 Li-S 电池的比较机理和动力学的原子尺度洞察
Na-S 和 Li-S 电池在取代普遍使用的锂离子电池方面处于领先地位。了解 Na-S 和 Li-S 之间的机械差异对于实现已开发技术向设计高性能正极材料的相互转移至关重要。锚固材料 (AMs) 需要克服性能限制因素,例如金属多硫化物 (M 2 S n , M = Na 和 Li, n= 1-8) 转化反应及其溶解到电解质中。本研究面临挑战,以批判性地了解 AM 在两种电池中的多硫化物化学中的作用。我们采用第一性原理密度泛函理论模拟来全面检查 M 2 Sn的吸附机制、硫还原反应 ( SRR ) 的动力学以及短链多硫化物在 Na-S 和 Li-S 电池上的催化分解。和钒 (V) 单原子催化剂嵌入 WSe 2 (V@WSe 2 ) 基板。我们发现原始WSe 2不能固定高阶M 2 S n;然而,V@WSe 2赋予足够的结合能来捕获高阶 M 2 S n。M 2 S n吸附强度的程度和 V@WSe 2的有效性在 Na-S 和 Li-S 系统之间有所不同。我们借助电荷转移、键强度和状态密度分析阐明了潜在的机械细节。重要的是,我们的模拟表明,在 V@WSe 2中,SRR 的限速步骤在 Li-S 中动力学更快,而放电终产物 M 2的氧化分解S 在 Na-S 电池中表现出加速动力学。这些发现对于理解 AM 在正极材料设计中的作用至关重要,以解决钠硫和锂硫电池(特别是金属硫电池)中的性能限制因素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号